Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
THERMAL PHYSICS
Содержание
- 1. THERMAL PHYSICS
- 2. Scan to read the message!
- 3. Learning Objectivesunderstand and describe principles of work of heat enginesrecall and apply
- 4. 2nd Law of ThermodynamicsA heat engine is
- 5. Reversible and Irreversible ProcessThermodynamic processes that occur
- 6. Reversible and Irreversible ProcessThe flow of heat
- 7. Reversible and Irreversible ProcessA system that undergoes
- 8. Reversible and Irreversible ProcessReversible processes are thus
- 9. Reversible and Irreversible Process
- 10. Reversible and Irreversible Process
- 11. Efficiency of Heat EngineThe efficiency (e or
- 12. Efficiency of Heat Engine
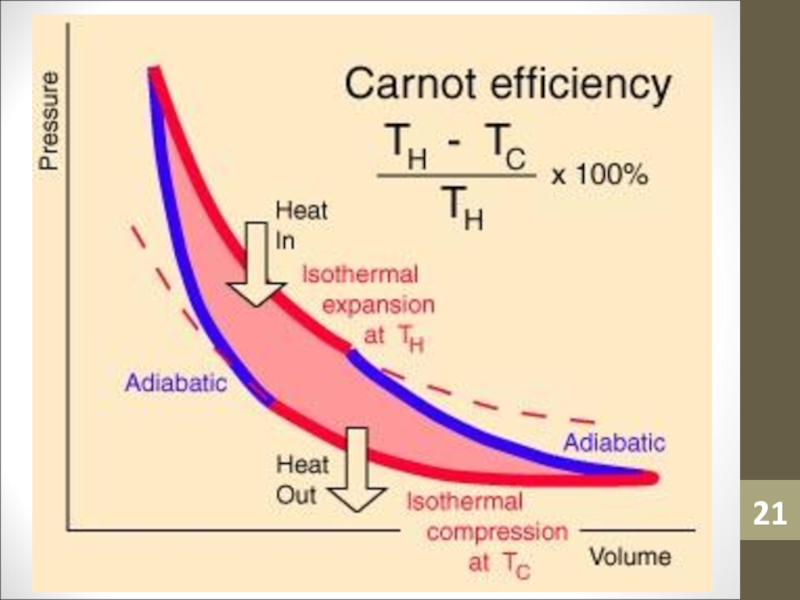
- 13. Carnot EngineTo see how to increase efficiency,
- 14. Carnot EngineNo Carnot engine actually exists but
- 15. Carnot EngineFor animation, please visit:http://science.sbcc.edu/~physics/flash/heatengines/Carnot%20cycle.htmlCarnot cycle explanation by Glen Research Center (NASA) please visit:http://www.grc.nasa.gov/WWW/K-12/airplane/carnot.html
- 16. Слайд 16
- 17. Carnot EngineThe first process performed on the
- 18. Carnot EngineThe second process performed on the
- 19. Carnot EngineThe third process performed on the
- 20. Carnot EngineThe fourth process performed on the
- 21. Слайд 21
- 22. Слайд 22
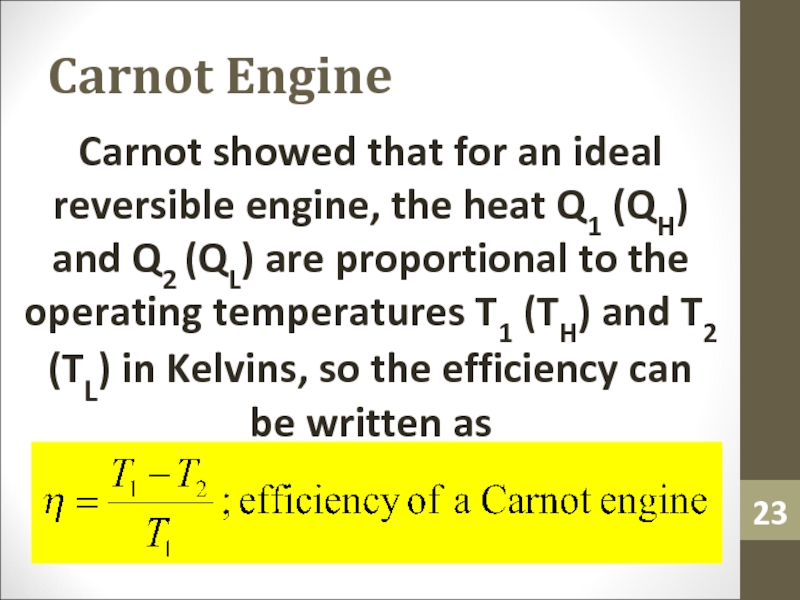
- 23. Carnot EngineCarnot showed that for an ideal
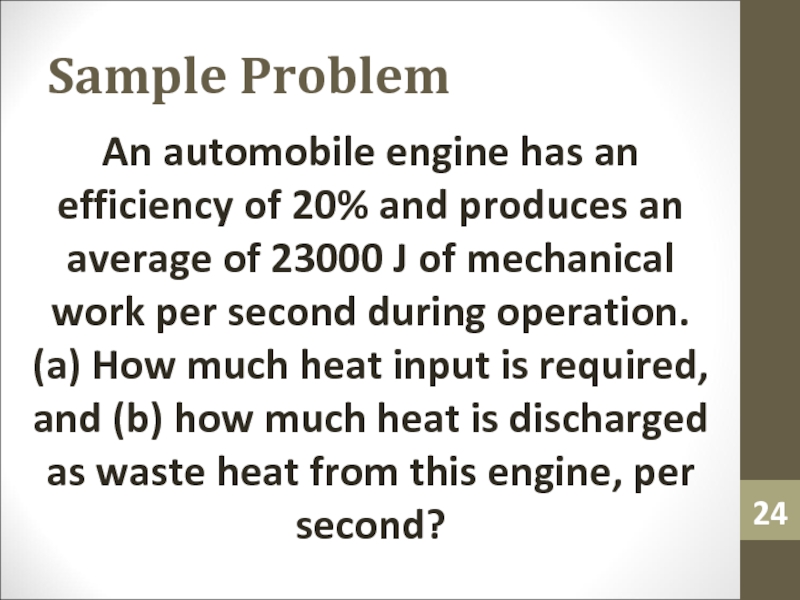
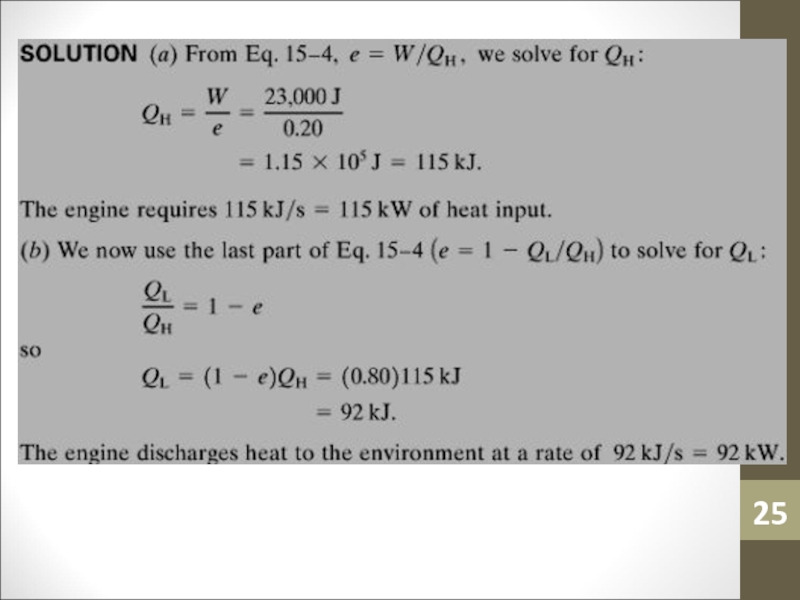
- 24. Sample ProblemAn automobile engine has an efficiency
- 25. Слайд 25
- 26. Sample ProblemA steam engine operates between 500
- 27. Слайд 27
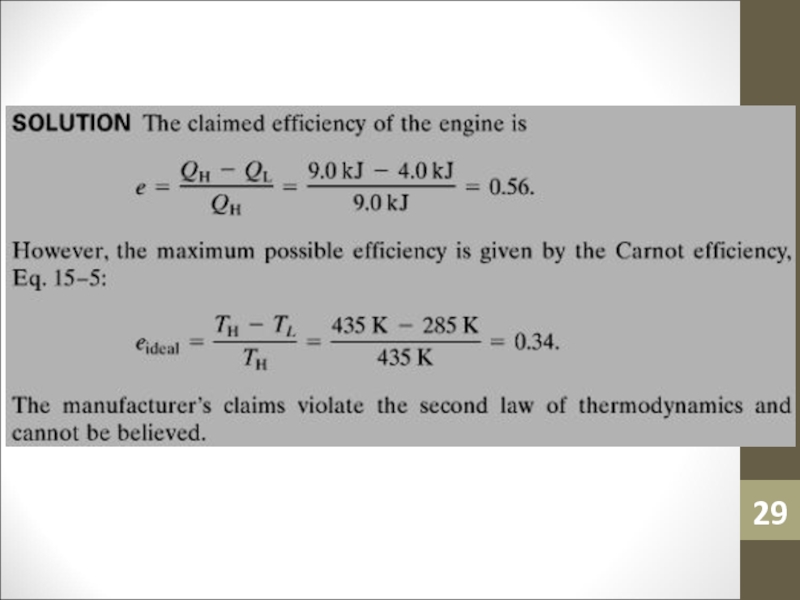
- 28. Sample ProblemAn engine manufacturer makes the following
- 29. Слайд 29
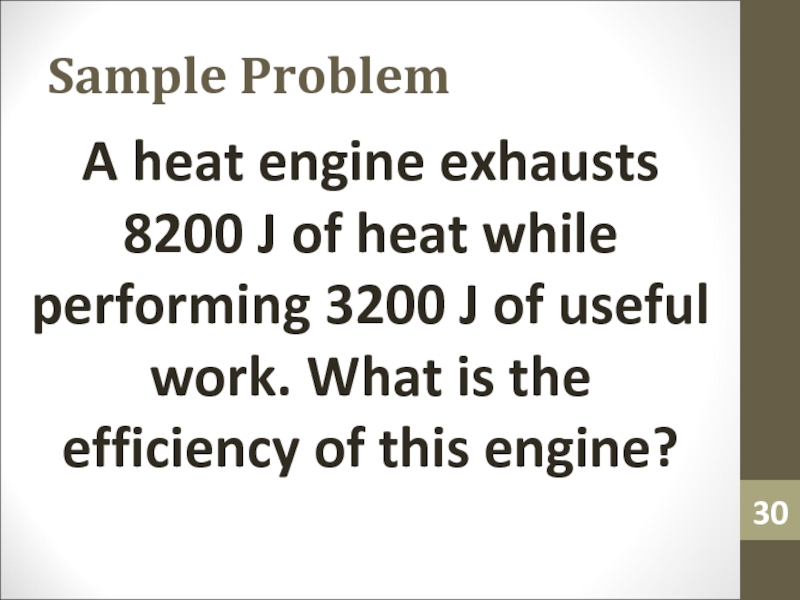
- 30. Sample ProblemA heat engine exhausts 8200 J
- 31. Слайд 31
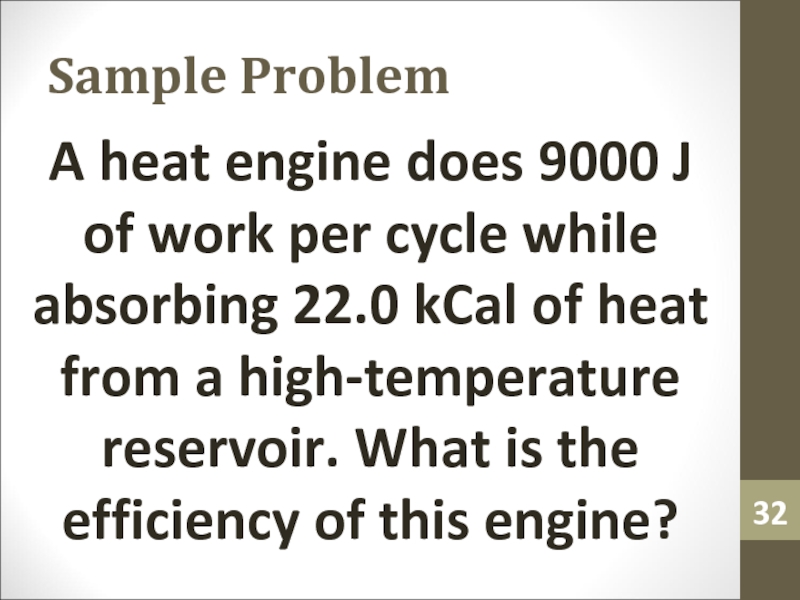
- 32. Sample ProblemA heat engine does 9000 J
- 33. Слайд 33
- 34. Sample ProblemWhat is the maximum efficiency of
- 35. Слайд 35
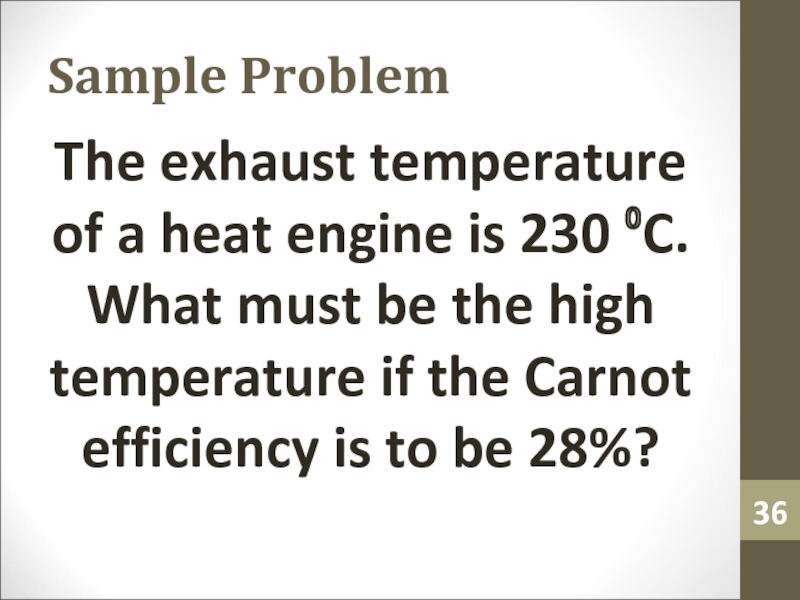
- 36. Sample ProblemThe exhaust temperature of a heat
- 37. Слайд 37
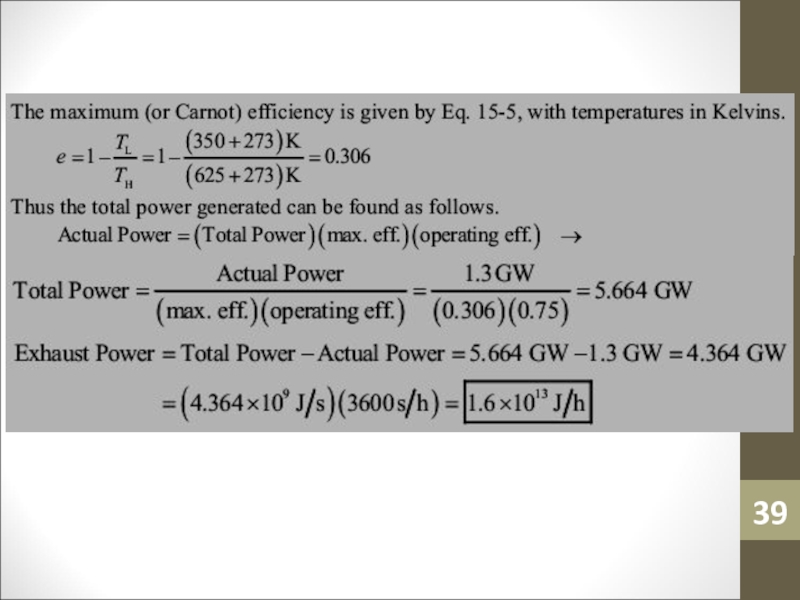
- 38. Sample ProblemA nuclear power plant operates at
- 39. Слайд 39
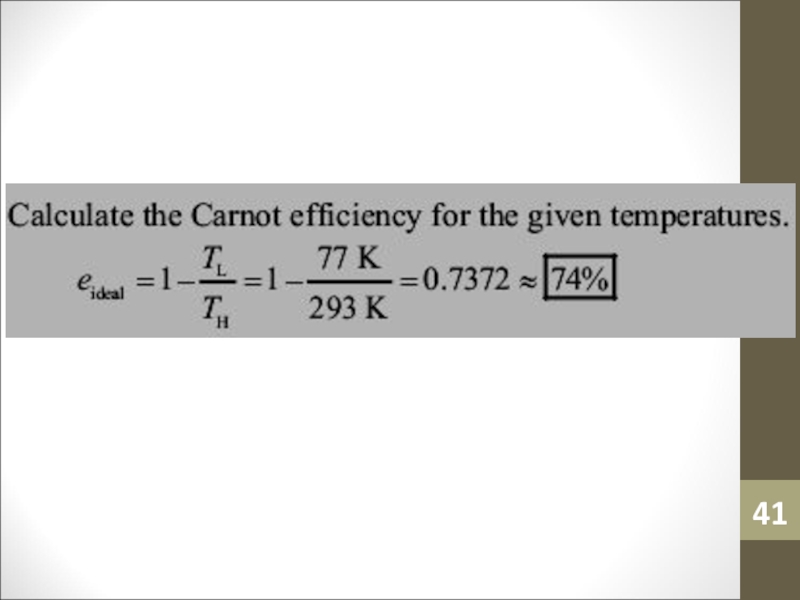
- 40. Sample ProblemIt is not necessary that a
- 41. Слайд 41
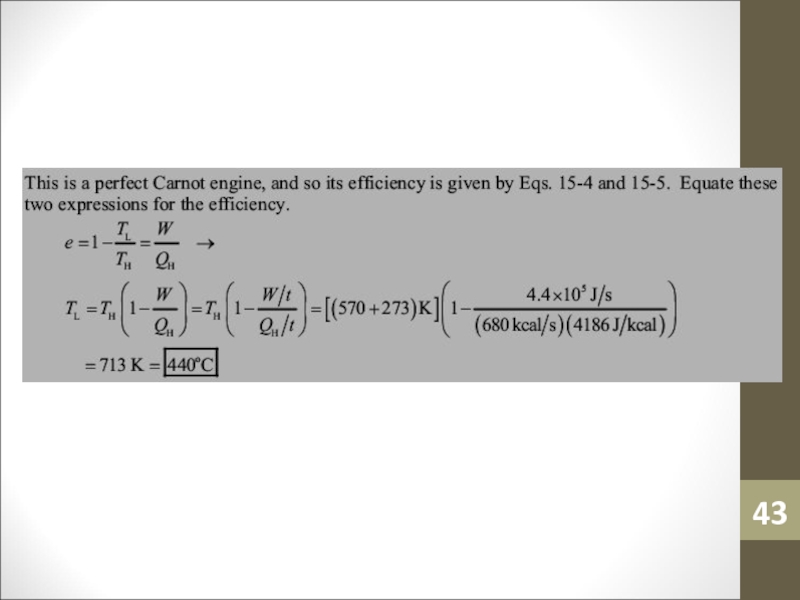
- 42. Sample ProblemA Carnot engine performs work at
- 43. Слайд 43
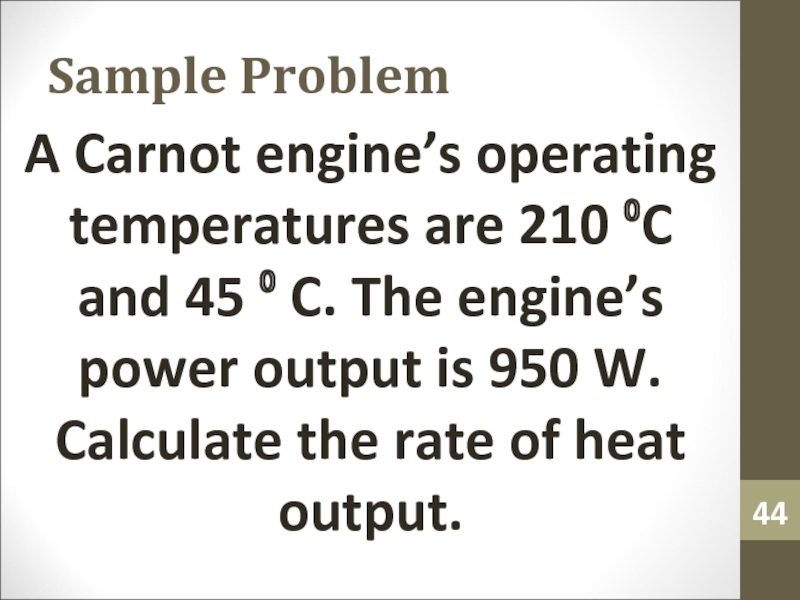
- 44. Sample ProblemA Carnot engine’s operating temperatures are
- 45. Слайд 45
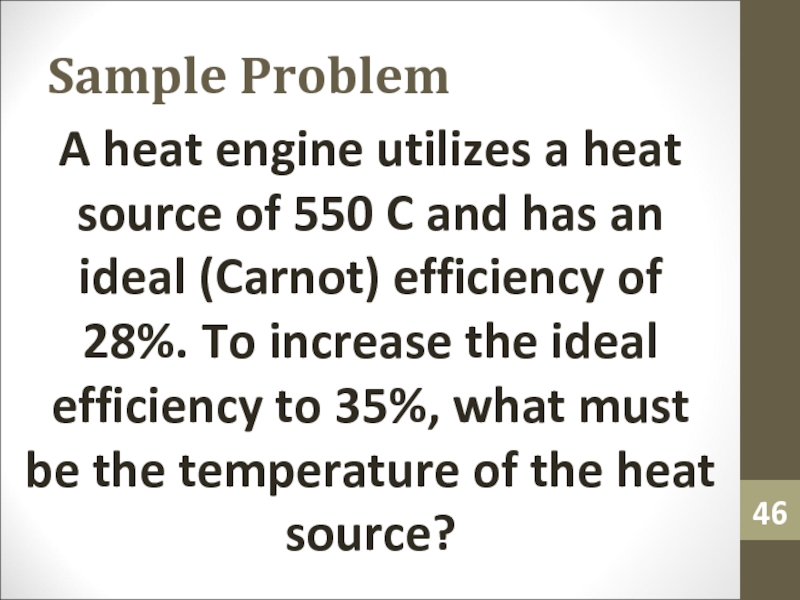
- 46. Sample ProblemA heat engine utilizes a heat
- 47. Слайд 47
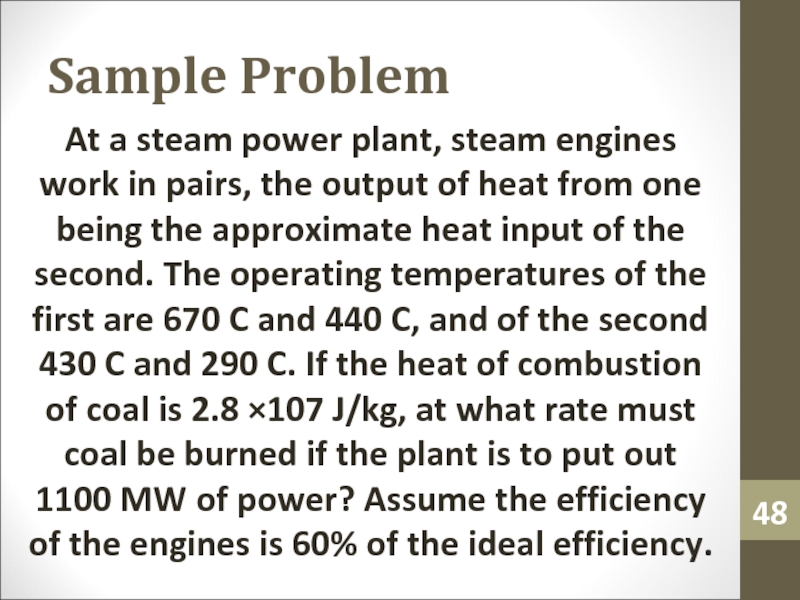
- 48. Sample ProblemAt a steam power plant, steam
- 49. Слайд 49
- 50. Скачать презентанцию
Scan to read the message!
Слайды и текст этой презентации
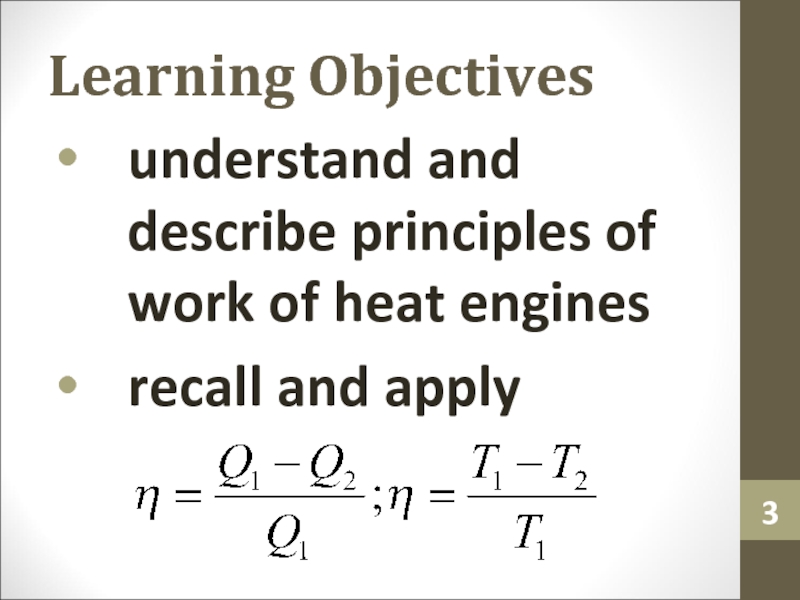
Слайд 3Learning Objectives
understand and describe principles of work of heat engines
recall
and apply
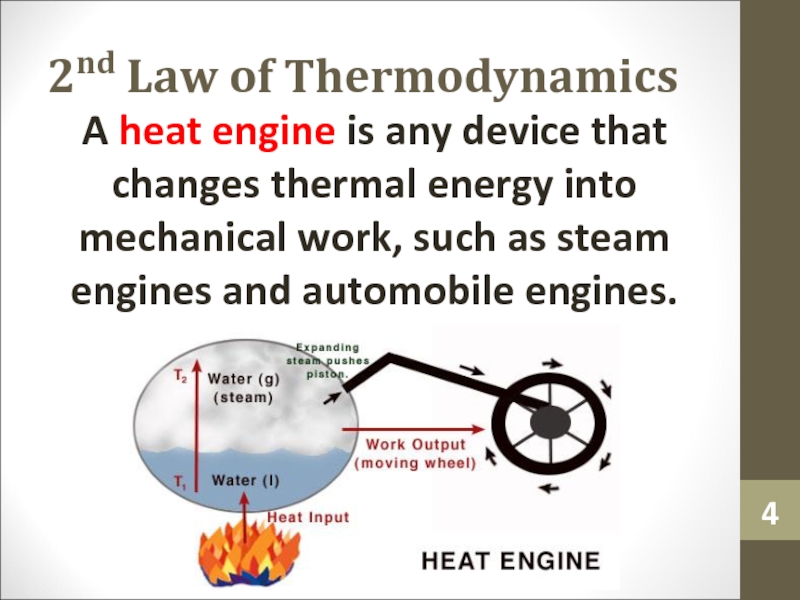
Слайд 42nd Law of Thermodynamics
A heat engine is any device that
changes thermal energy into mechanical work, such as steam engines
and automobile engines.Слайд 5Reversible and Irreversible Process
Thermodynamic processes that occur in nature are
all irreversible process. These are processes that proceed spontaneously in
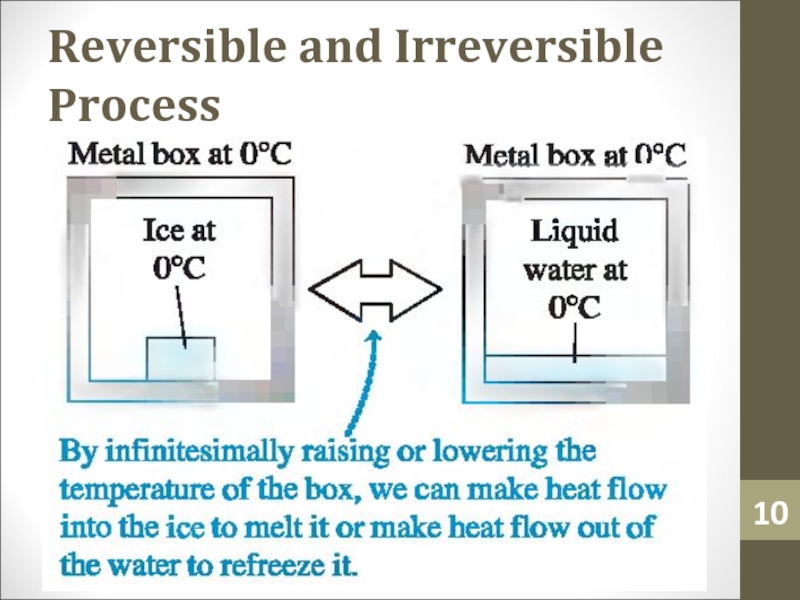
one direction but not the other.Слайд 6Reversible and Irreversible Process
The flow of heat from a hot
body to a cooler body is irreversible.
Sliding a book across
a table converts mechanical energy into heat by friction; this is irreversible, for no one has ever observe the reverse process.Слайд 7Reversible and Irreversible Process
A system that undergoes an “idealized” reversible
process is always very close to being in thermodynamic equilibrium
within itself and with its surroundings.Слайд 8Reversible and Irreversible Process
Reversible processes are thus equilibrium processes, with
the system always in thermodynamic equilibrium.
A reversible process is an
idealization that can never be precisely attained in real world.Слайд 11Efficiency of Heat Engine
The efficiency (e or η) of any
heat engine can be identified as the ratio of the
work (W) it does to the heat input at the high temperature (QH or Q1).Слайд 13Carnot Engine
To see how to increase efficiency, the French scientist
Sadi Carnot (1796 – 1832) examined the characteristics of an
ideal engine (now called Carnot Engine).Слайд 14Carnot Engine
No Carnot engine actually exists but as a theoretical
idea it played an important role in the development of
thermodynamics.The idealized Carnot engine consisted of four (4) processes done in a cycle, two of which are adiabatic (Q = 0) and two are isothermal (ΔT = 0).
Слайд 15Carnot Engine
For animation, please visit:
http://science.sbcc.edu/~physics/flash/heatengines/Carnot%20cycle.html
Carnot cycle explanation by Glen Research
Center (NASA) please visit:
http://www.grc.nasa.gov/WWW/K-12/airplane/carnot.html
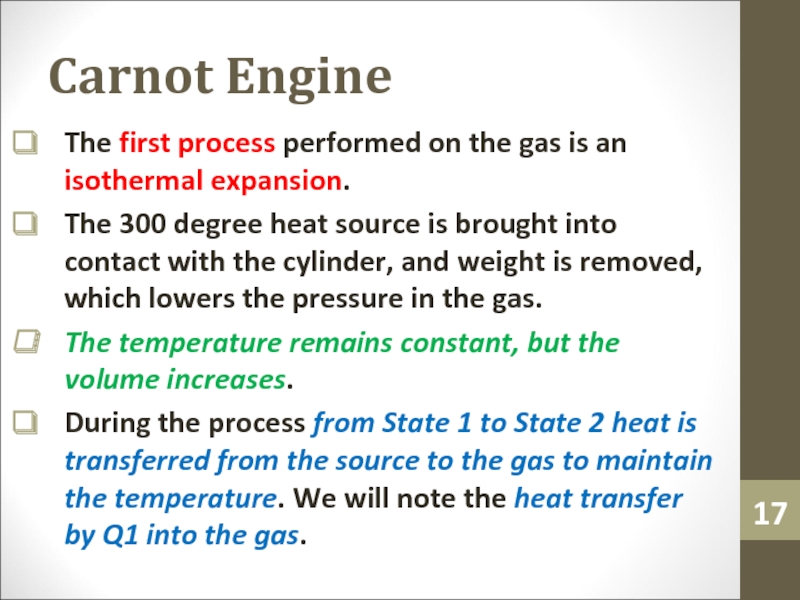
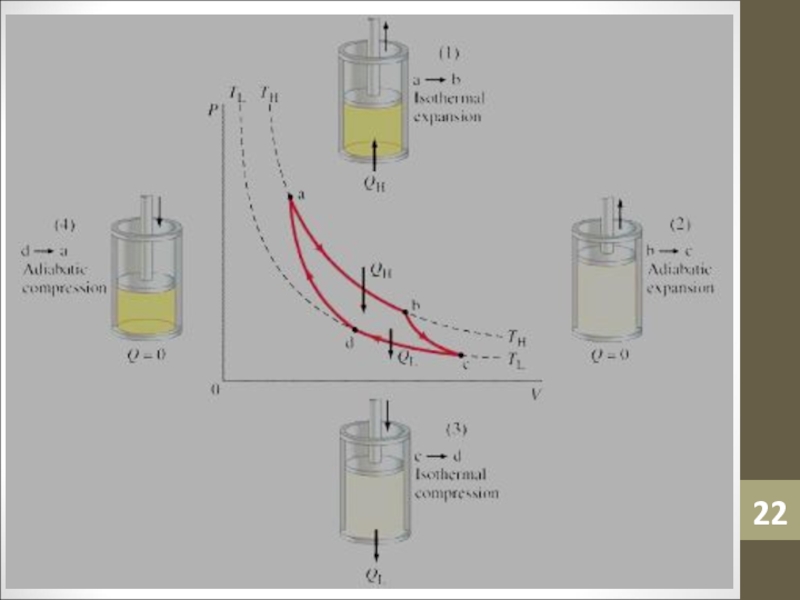
Слайд 17Carnot Engine
The first process performed on the gas is an
isothermal expansion.
The 300 degree heat source is brought into contact
with the cylinder, and weight is removed, which lowers the pressure in the gas.The temperature remains constant, but the volume increases.
During the process from State 1 to State 2 heat is transferred from the source to the gas to maintain the temperature. We will note the heat transfer by Q1 into the gas.
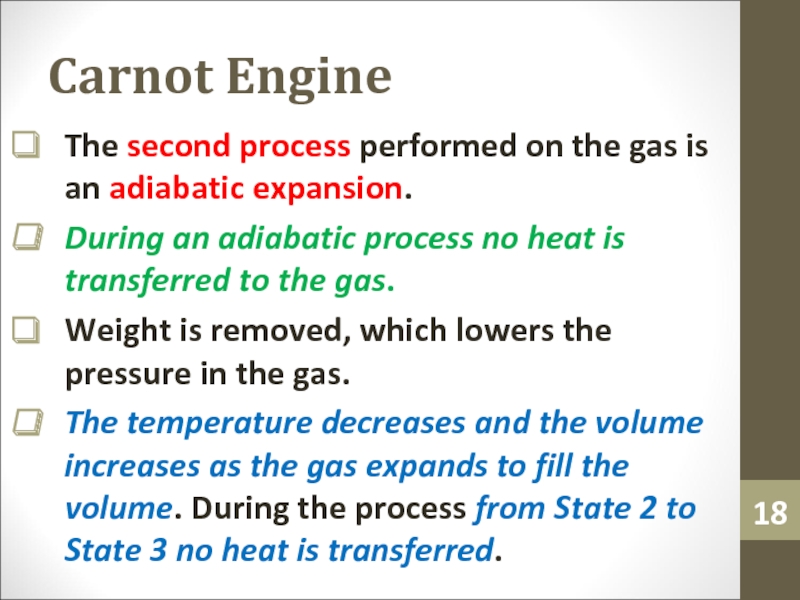
Слайд 18Carnot Engine
The second process performed on the gas is an
adiabatic expansion.
During an adiabatic process no heat is transferred to
the gas.Weight is removed, which lowers the pressure in the gas.
The temperature decreases and the volume increases as the gas expands to fill the volume. During the process from State 2 to State 3 no heat is transferred.
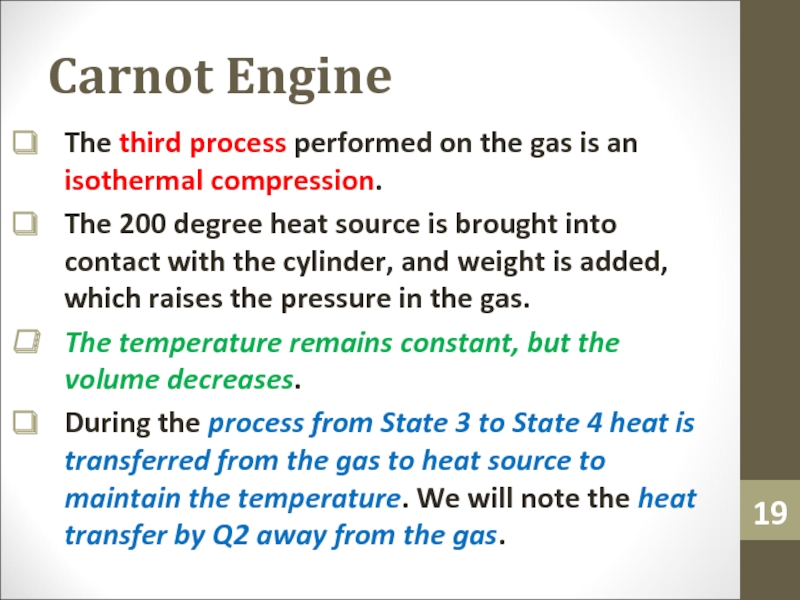
Слайд 19Carnot Engine
The third process performed on the gas is an
isothermal compression.
The 200 degree heat source is brought into contact
with the cylinder, and weight is added, which raises the pressure in the gas.The temperature remains constant, but the volume decreases.
During the process from State 3 to State 4 heat is transferred from the gas to heat source to maintain the temperature. We will note the heat transfer by Q2 away from the gas.
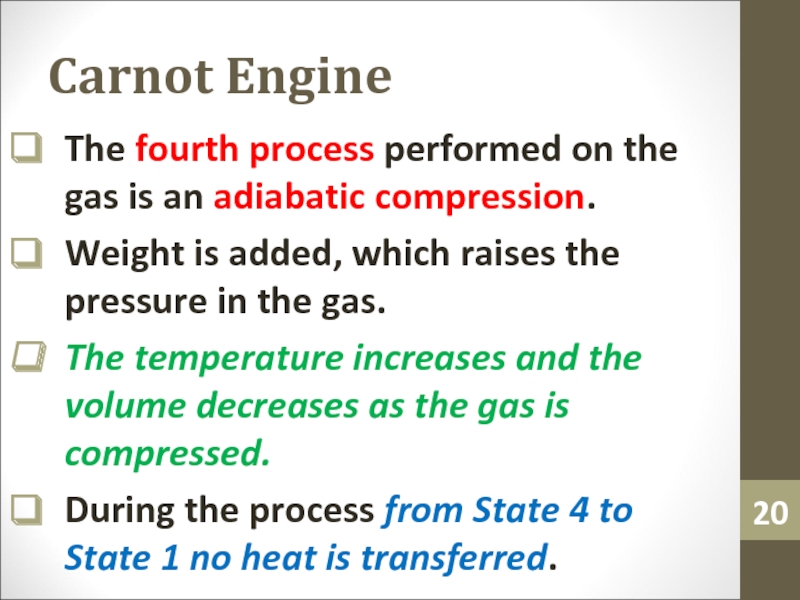
Слайд 20Carnot Engine
The fourth process performed on the gas is an
adiabatic compression.
Weight is added, which raises the pressure in the
gas.The temperature increases and the volume decreases as the gas is compressed.
During the process from State 4 to State 1 no heat is transferred.