Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
THERMAL PHYSICS
Содержание
- 1. THERMAL PHYSICS
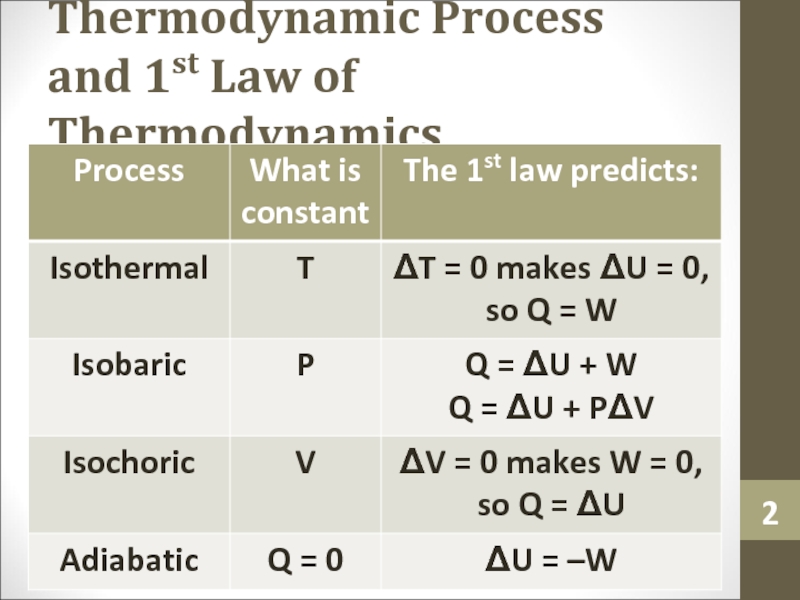
- 2. Thermodynamic Process and 1st Law of Thermodynamics
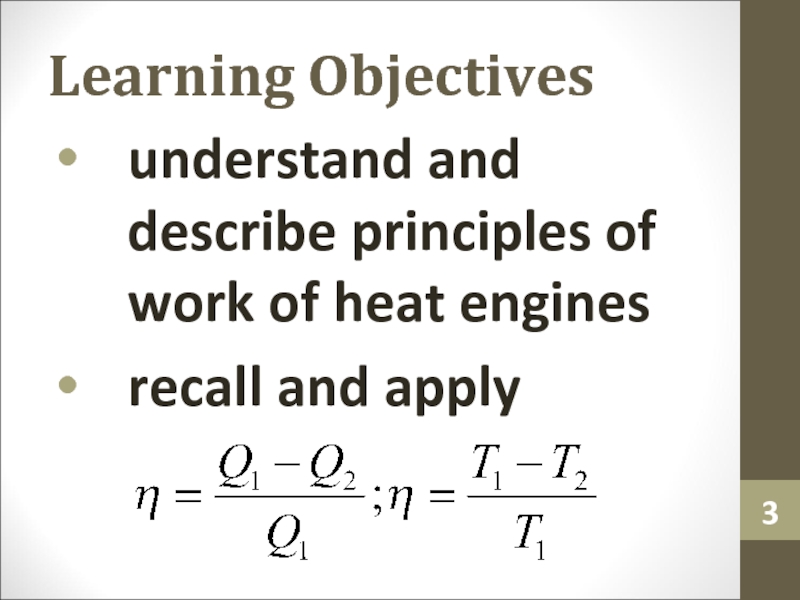
- 3. Learning Objectivesunderstand and describe principles of work of heat enginesrecall and apply
- 4. 2nd Law of ThermodynamicsThere are many processes
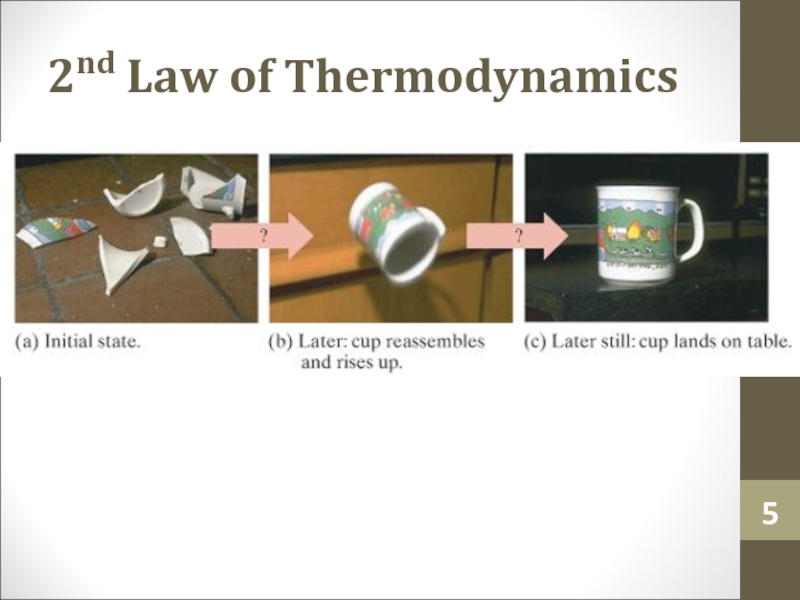
- 5. 2nd Law of Thermodynamics
- 6. 2nd Law of ThermodynamicsIf you put a
- 7. 2nd Law of ThermodynamicsCoffee cups and glasses
- 8. 2nd Law of ThermodynamicsThe air in a
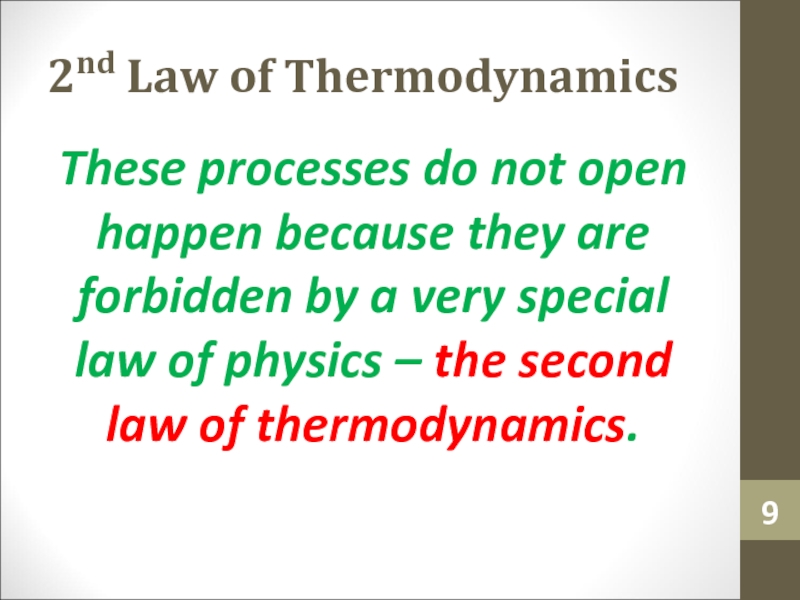
- 9. 2nd Law of ThermodynamicsThese processes do not
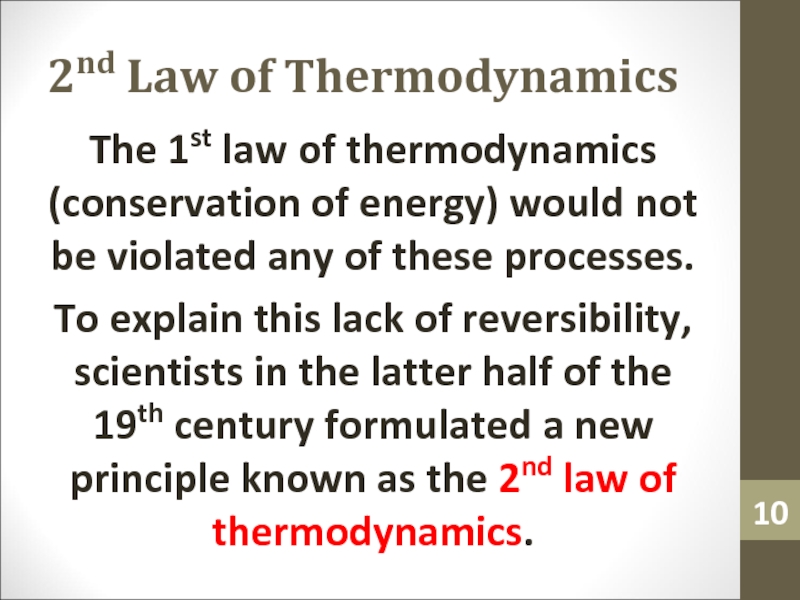
- 10. 2nd Law of ThermodynamicsThe 1st law of
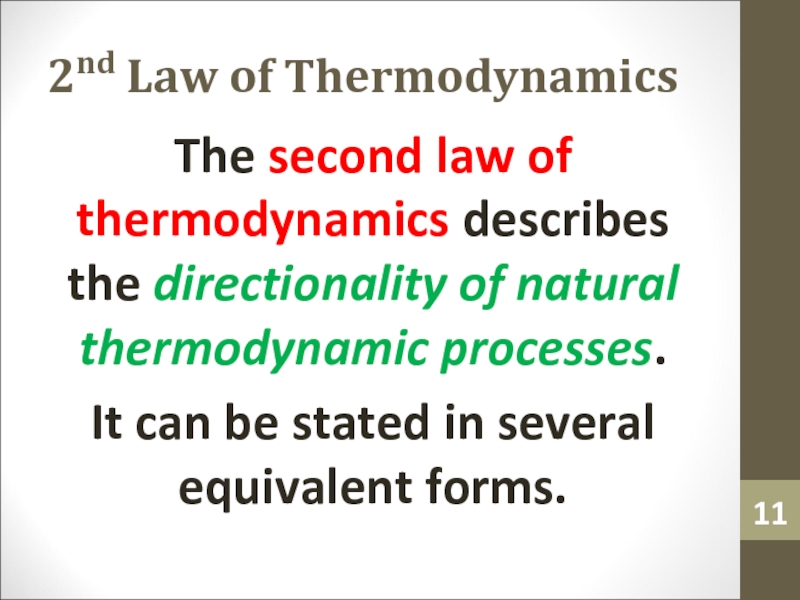
- 11. 2nd Law of ThermodynamicsThe second law of
- 12. 2nd Law of ThermodynamicsAccording to Rudolf J.E.
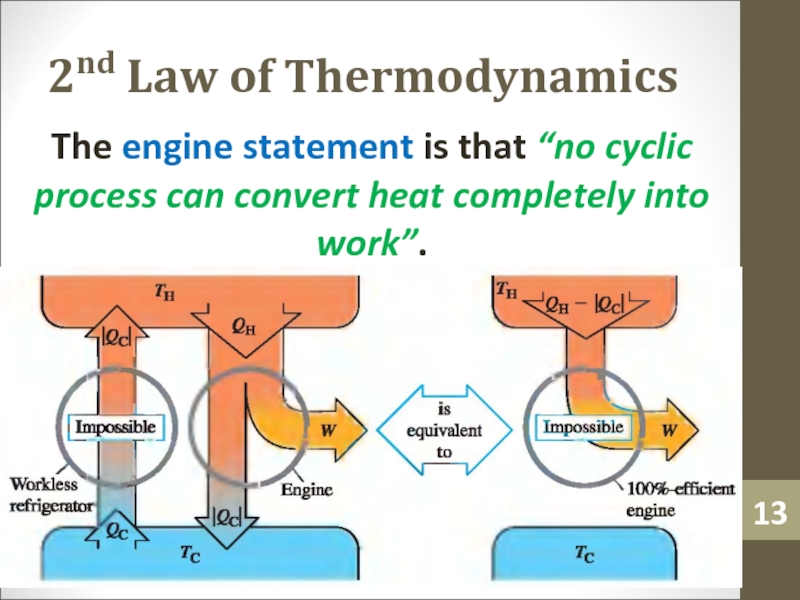
- 13. 2nd Law of ThermodynamicsThe engine statement is
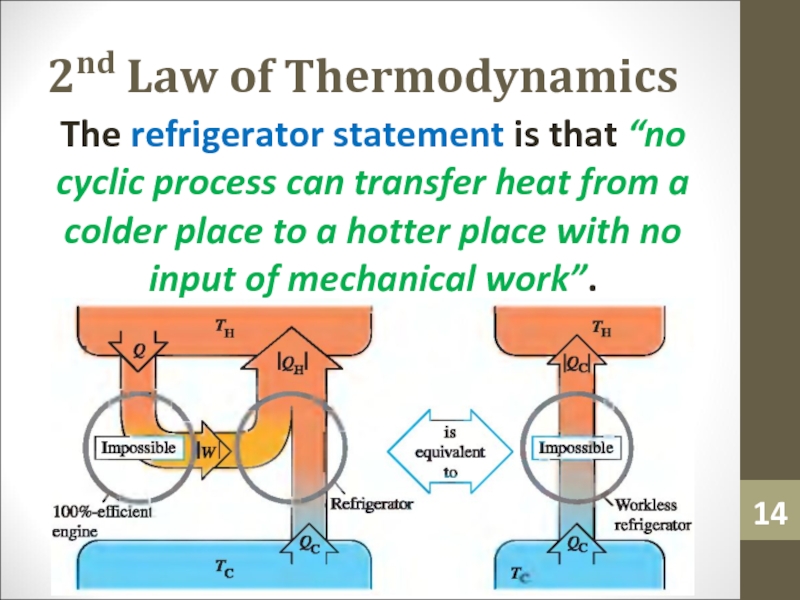
- 14. 2nd Law of ThermodynamicsThe refrigerator statement is
- 15. 2nd Law of ThermodynamicsThe development of a
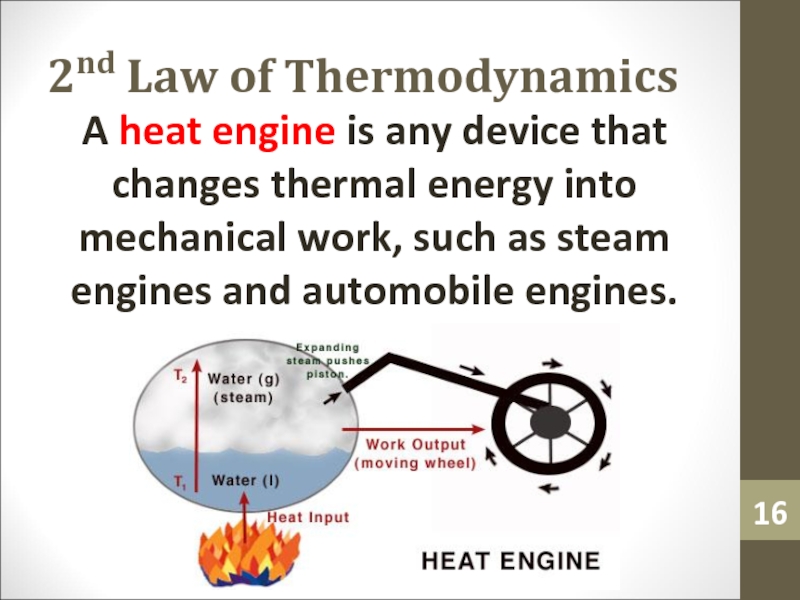
- 16. 2nd Law of ThermodynamicsA heat engine is
- 17. 2nd Law of ThermodynamicsThe second law of
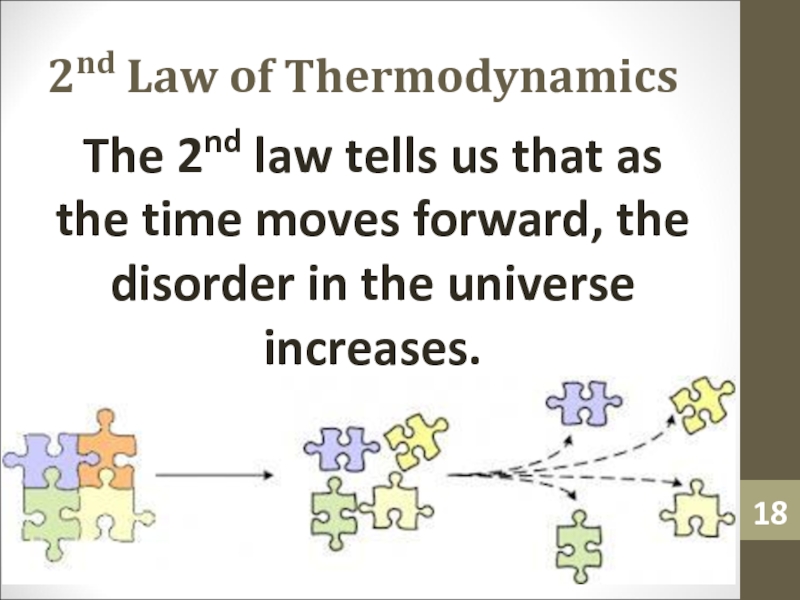
- 18. 2nd Law of ThermodynamicsThe 2nd law tells
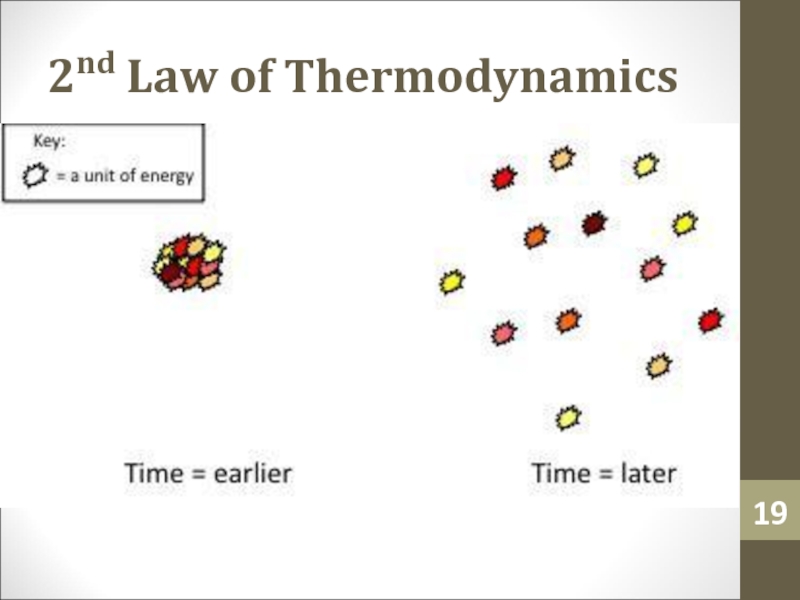
- 19. 2nd Law of Thermodynamics
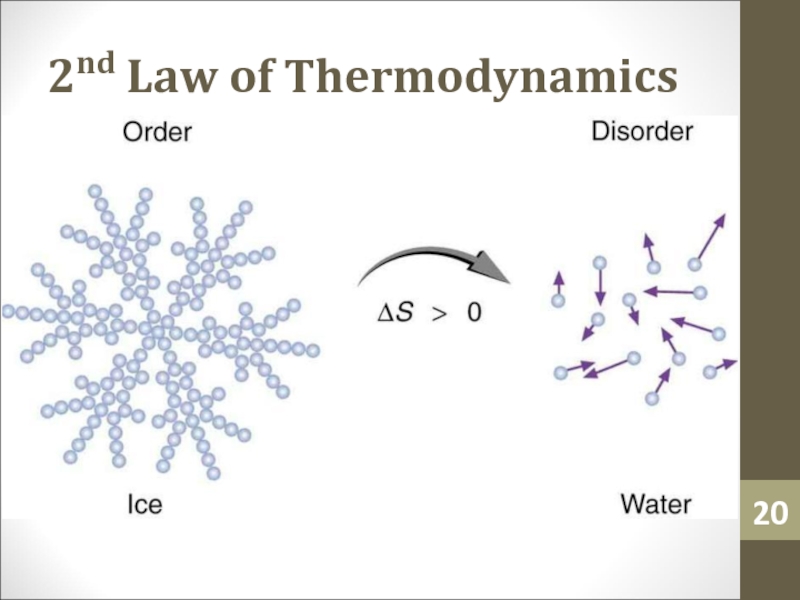
- 20. 2nd Law of Thermodynamics
- 21. Reversible and Irreversible ProcessThermodynamic processes that occur
- 22. Reversible and Irreversible ProcessThe flow of heat
- 23. Reversible and Irreversible ProcessA system that undergoes
- 24. Reversible and Irreversible ProcessReversible processes are thus
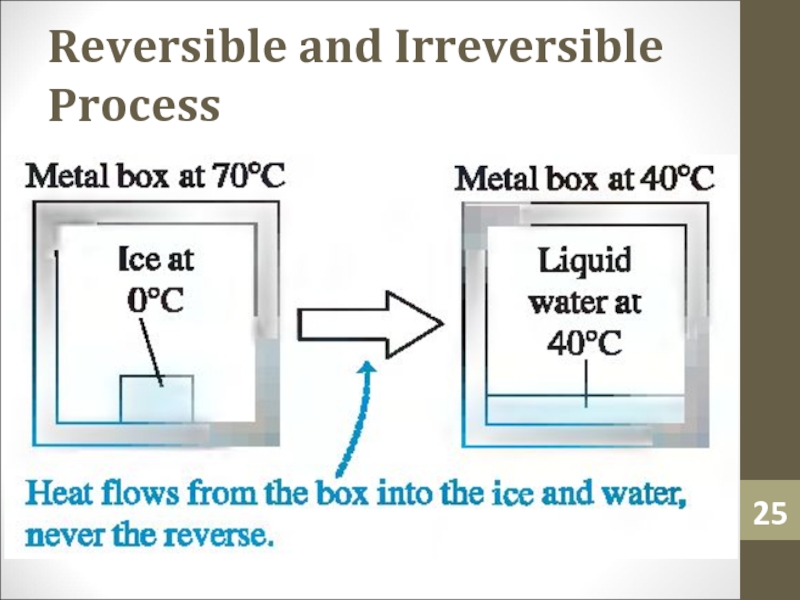
- 25. Reversible and Irreversible Process
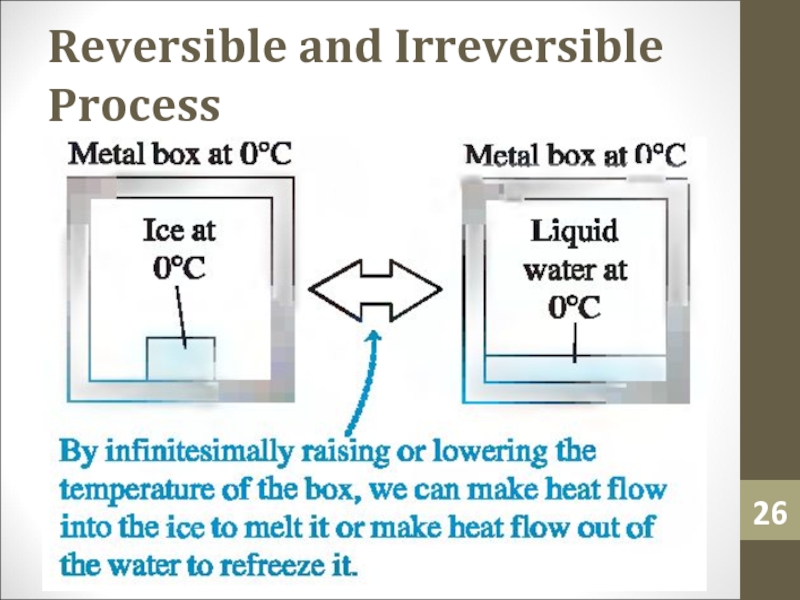
- 26. Reversible and Irreversible Process
- 27. Скачать презентанцию
Thermodynamic Process and 1st Law of Thermodynamics
Слайды и текст этой презентации
Слайд 3Learning Objectives
understand and describe principles of work of heat engines
recall
and apply
Слайд 42nd Law of Thermodynamics
There are many processes in thermodynamics that
are consistent with the first law but are nonetheless impossible.
Слайд 62nd Law of Thermodynamics
If you put a layer of salt
in a jar and cover it with a layer of
similar-sized grains of pepper, when you shake it you get a thorough mixture. But no matter how long you shake it, the mixture does not separate into layers again.Слайд 72nd Law of Thermodynamics
Coffee cups and glasses break spontaneously if
you drop them. But they don’t go back together spontaneously.
The
spontaneous (without the action of another agent) transfer of thermal energy from a cold body to hotter bodyСлайд 82nd Law of Thermodynamics
The air in a room suddenly occupying
just one half of the room and leaving the other
half empty.A glass of water at room temperature suddenly freezing, causing the temperature of the room to rise