Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
THERMAL PHYSICS
Содержание
- 1. THERMAL PHYSICS
- 2. Learning Objectivesunderstand and apply first law of
- 3. Terms to Remember!ThermodynamicsThermodynamic SystemSurroundingsHeatWorkInternal Energy
- 4. ThermodynamicsThermodynamics is the macroscopic study of the
- 5. Thermodynamic SystemThermodynamic System is a macroscopic aspect
- 6. SurroundingsSurroundings is everything in the problem outside
- 7. Surroundings
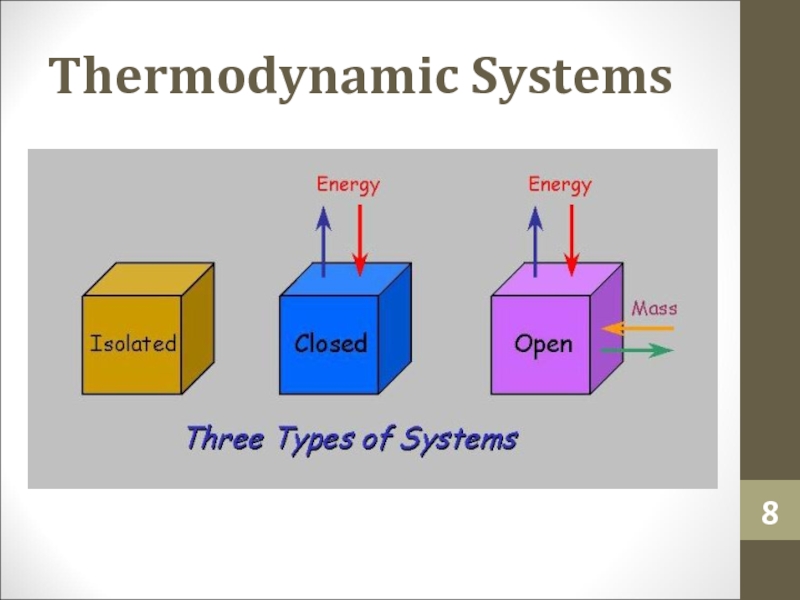
- 8. Thermodynamic Systems
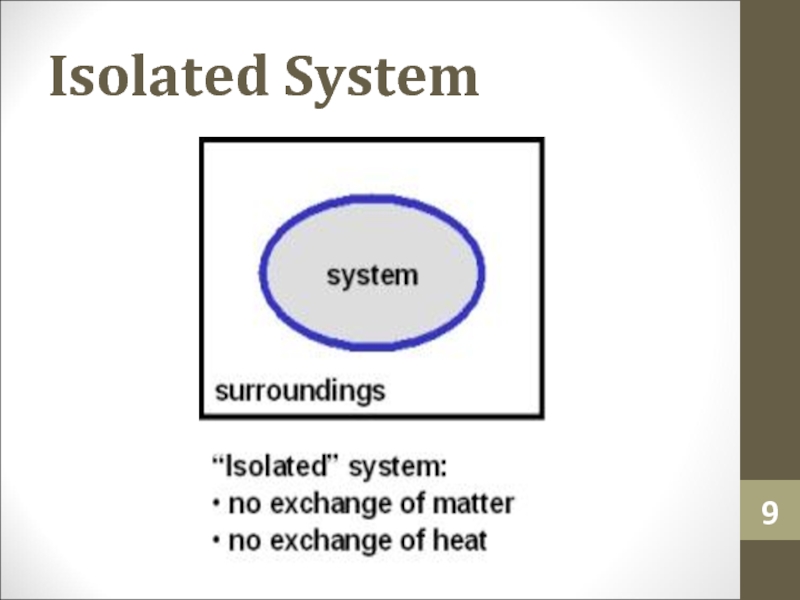
- 9. Isolated System
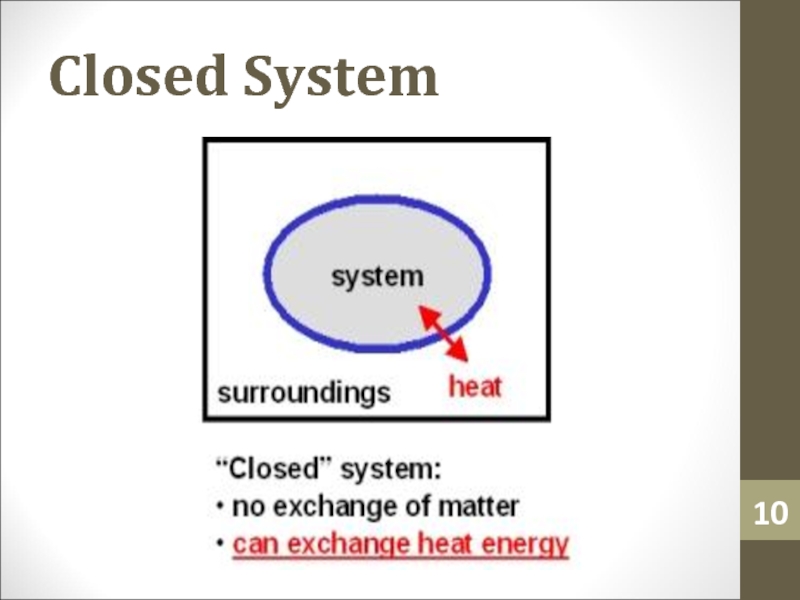
- 10. Closed System
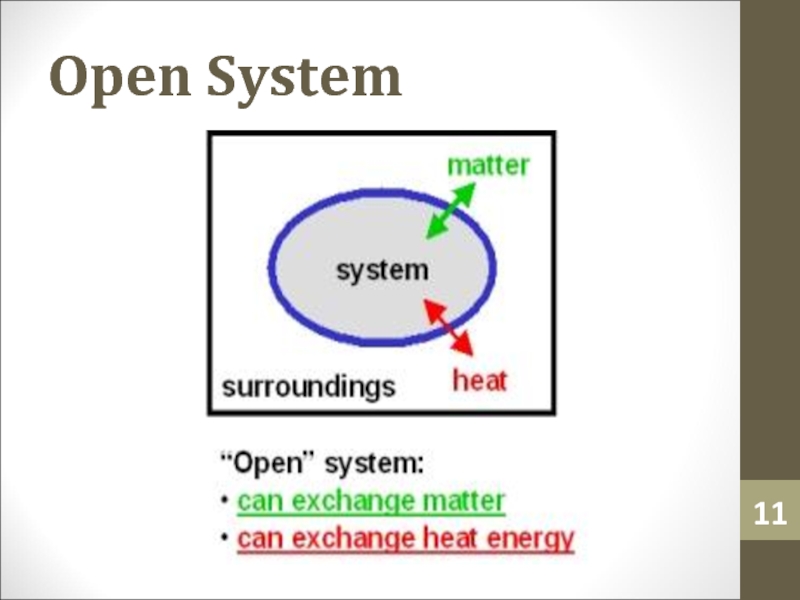
- 11. Open System
- 12. HeatHeat (Q) is an amount of thermal
- 13. Heat Flow (Review)
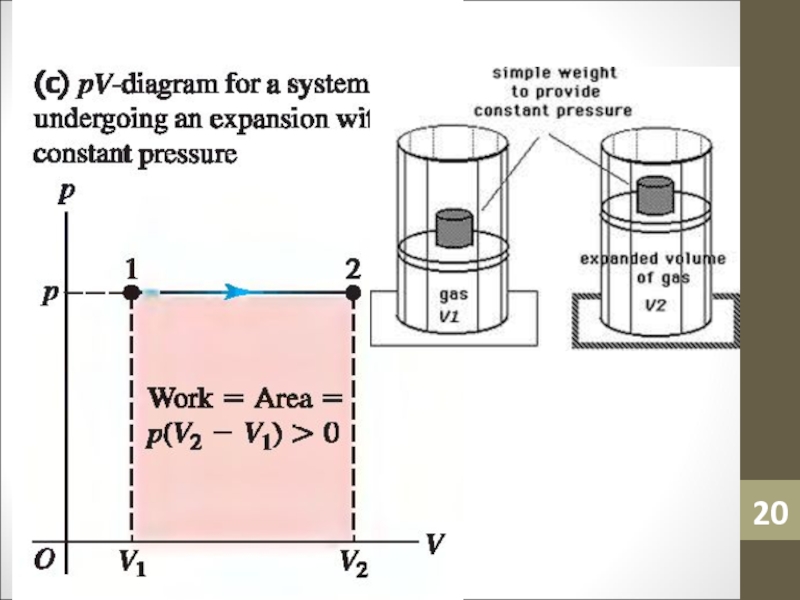
- 14. WorkWork (W) is simply a macroscopic transfer of energy from the gas to the surroundings.
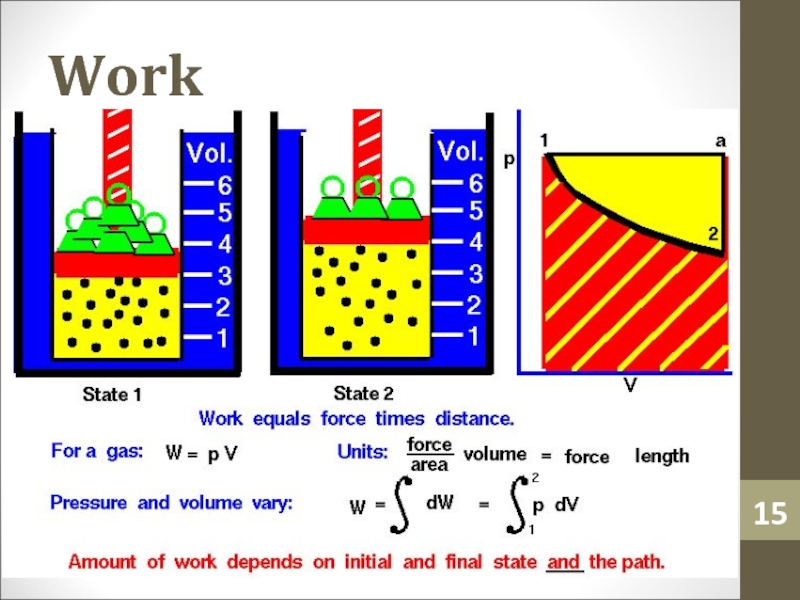
- 15. Work
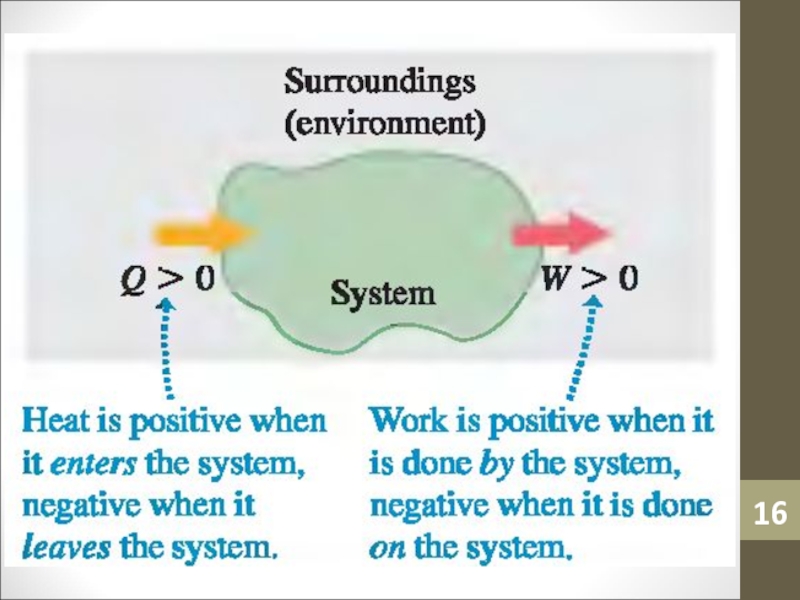
- 16. Слайд 16
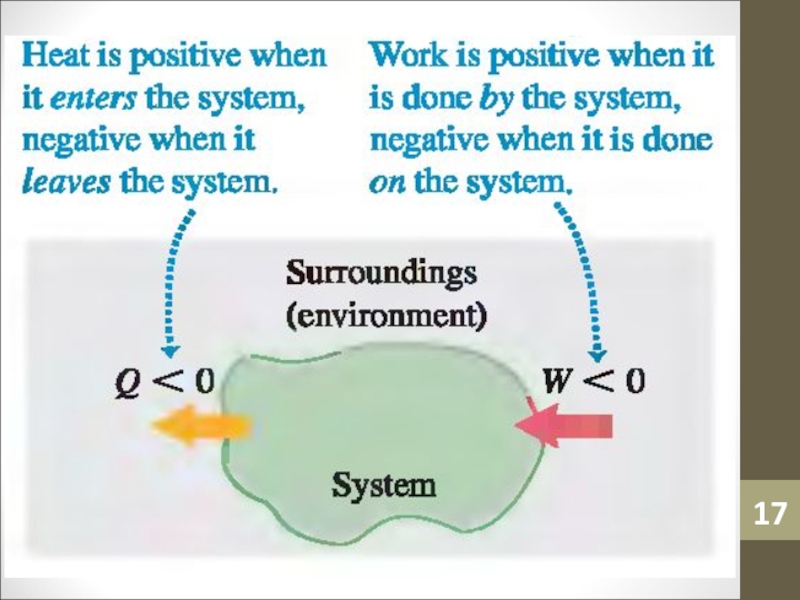
- 17. Слайд 17
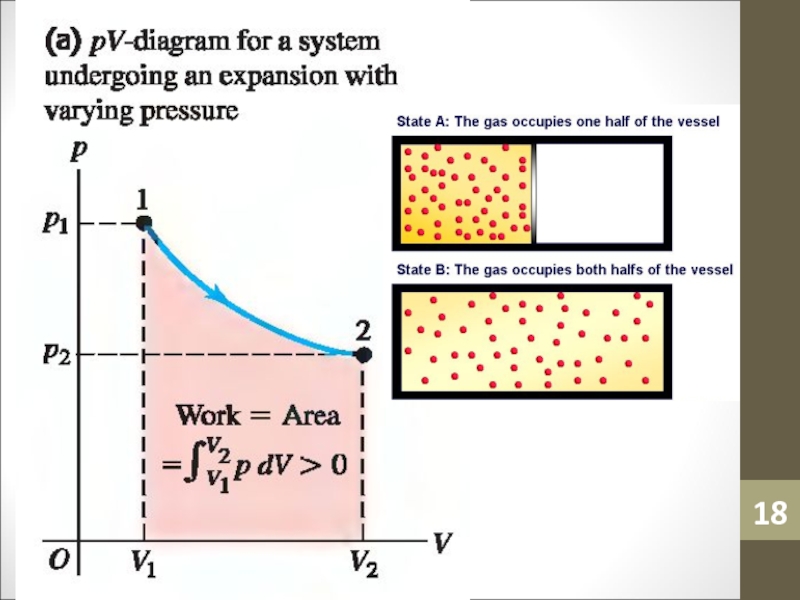
- 18. Слайд 18
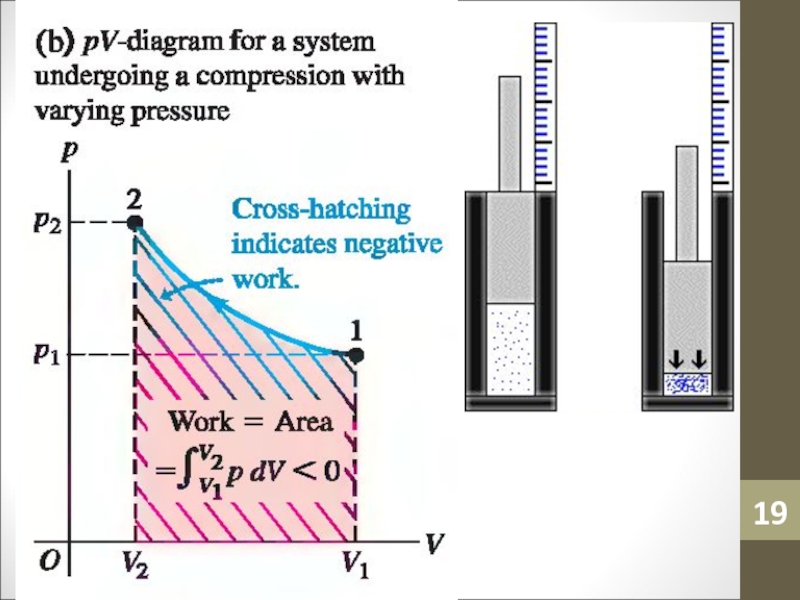
- 19. Слайд 19
- 20. Слайд 20
- 21. Change in Internal EnergyChange in Internal Energy
- 22. Change in Internal EnergyThe change in internal
- 23. 1st Law of ThermodynamicsThe First Law of
- 24. 1st Law of ThermodynamicsSince Q and W
- 25. 1st Law of ThermodynamicsThe internal energy of
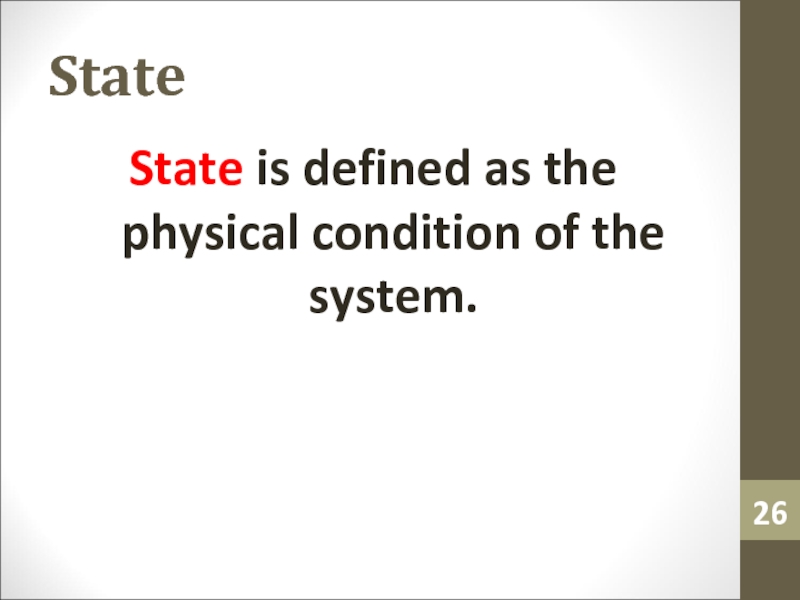
- 26. StateState is defined as the physical condition of the system.
- 27. Important NoteA given system at any moment
- 28. Important NoteRather, when work is done on
- 29. Important NoteThus, work and heat are involved
- 30. State VariablesQuantities which describe the state of
- 31. Sample Problem2500 J of heat is added
- 32. Sample Problem2500 J of heat is added
- 33. Isothermal Process and 1st Law of ThermodynamicsTo
- 34. Isothermal Process and 1st Law of ThermodynamicsConsider
- 35. Isothermal Process and 1st Law of ThermodynamicsThe
- 36. Isothermal Process and 1st Law of ThermodynamicsEach
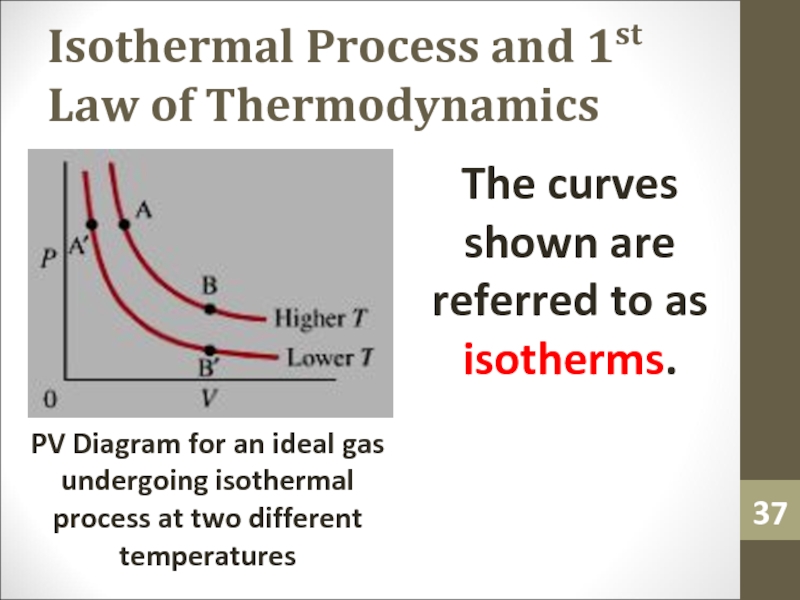
- 37. Isothermal Process and 1st Law of ThermodynamicsThe
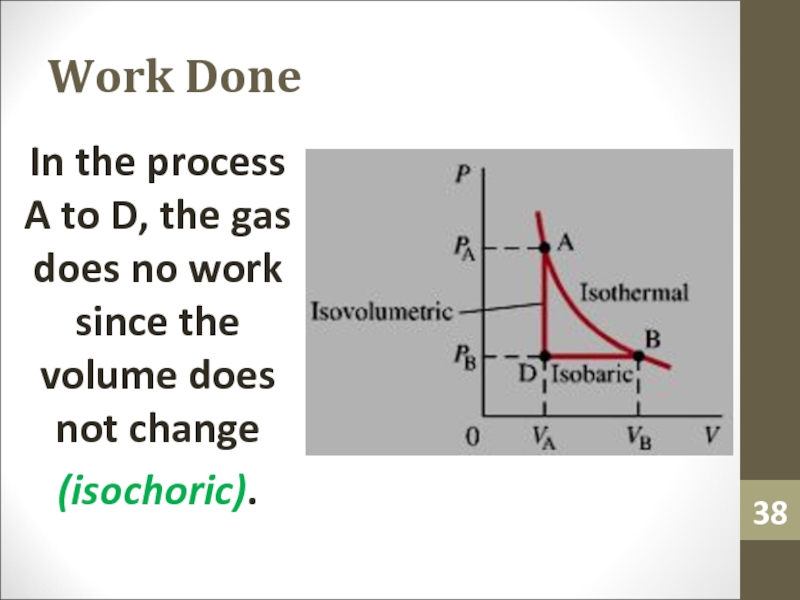
- 38. Work DoneIn the process A to D,
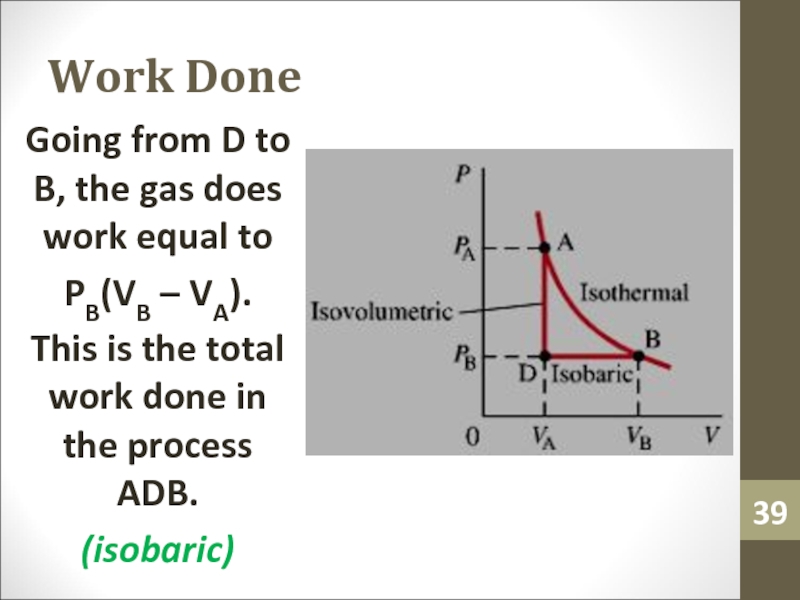
- 39. Work DoneGoing from D to B, the
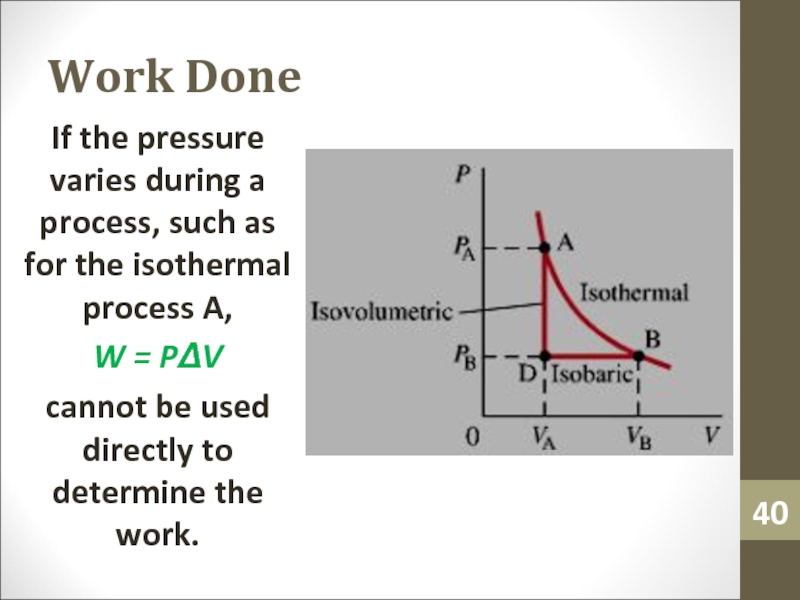
- 40. Work DoneIf the pressure varies during a
- 41. Work DoneThe calculation of work done (WAB)
- 42. Adiabatic Process and 1st Law of ThermodynamicsAn
- 43. Adiabatic Process and 1st Law of ThermodynamicsThe
- 44. Adiabatic Process and 1st Law of ThermodynamicsA
- 45. Adiabatic Process and 1st Law of ThermodynamicsSince
- 46. Adiabatic Process and 1st Law of ThermodynamicsIn
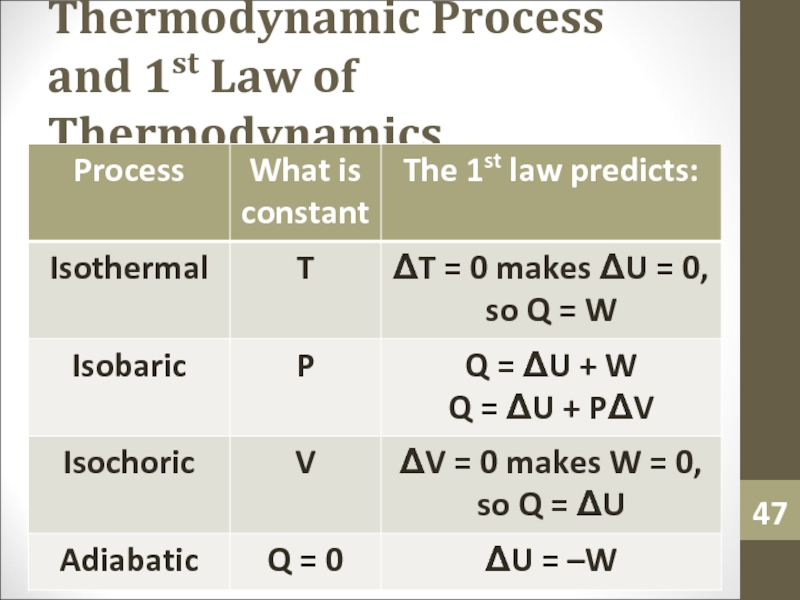
- 47. Thermodynamic Process and 1st Law of Thermodynamics
- 48. Sample Problem:An ideal gas is slowly compressed
- 49. Слайд 49
- 50. Sample Problem:In an engine, 0.25 moles of
- 51. Слайд 51
- 52. Sample Problem:A gas in a container with
- 53. Слайд 53
- 54. Sample Problem:A gas expands adiabatically. Will its temperature increase or decrease?
- 55. Слайд 55
- 56. Sample Problem:A monatomic gas is kept at
- 57. Слайд 57
- 58. 2nd Law of ThermodynamicsThere are many processes
- 59. 2nd Law of ThermodynamicsIf you put a
- 60. 2nd Law of ThermodynamicsCoffee cups and glasses
- 61. 2nd Law of ThermodynamicsThe air in a
- 62. 2nd Law of ThermodynamicsThese processes do not
- 63. 2nd Law of ThermodynamicsThe 1st law of
- 64. 2nd Law of ThermodynamicsThe second law of
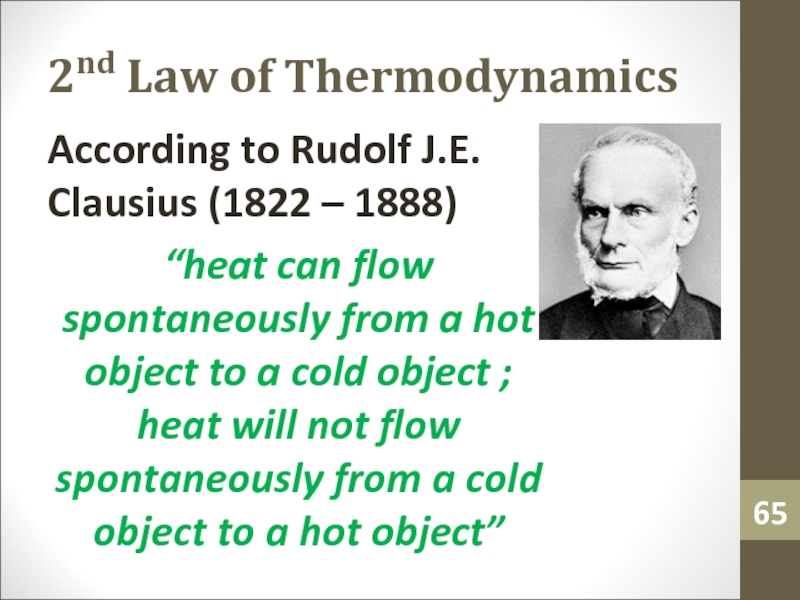
- 65. 2nd Law of ThermodynamicsAccording to Rudolf J.E.
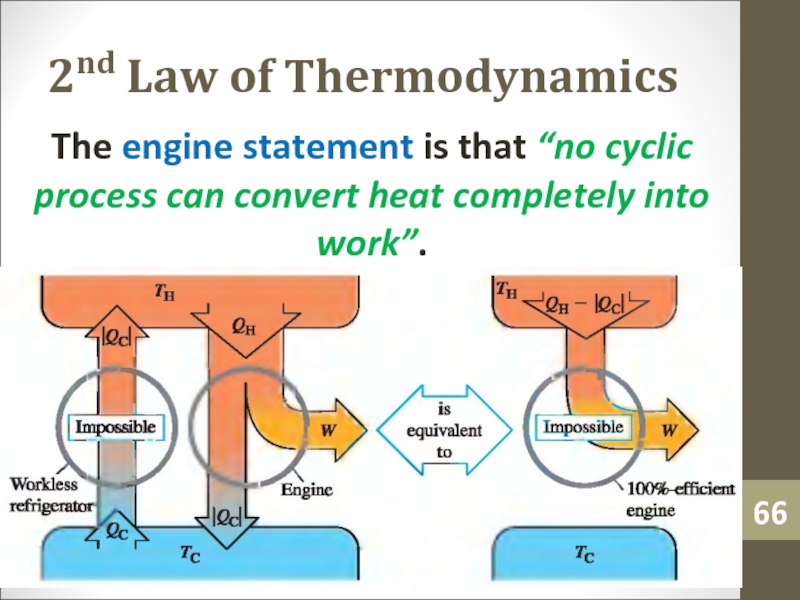
- 66. 2nd Law of ThermodynamicsThe engine statement is
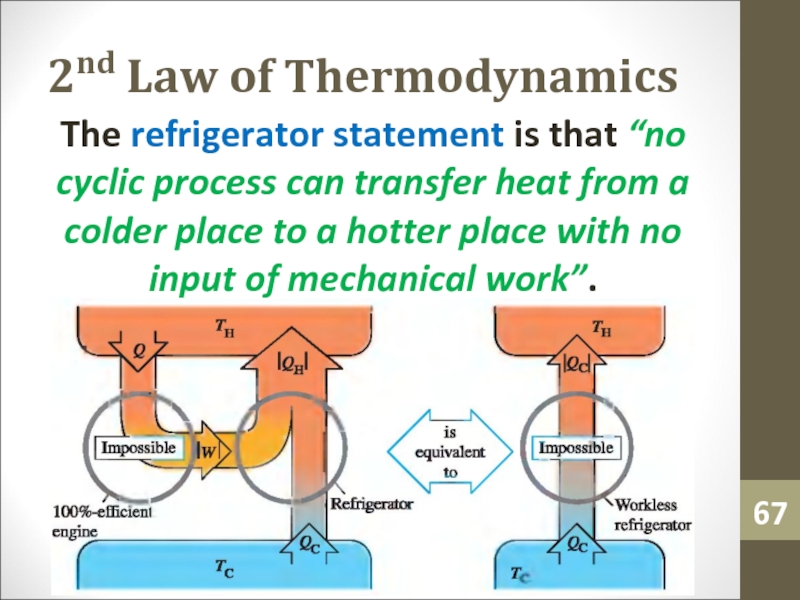
- 67. 2nd Law of ThermodynamicsThe refrigerator statement is
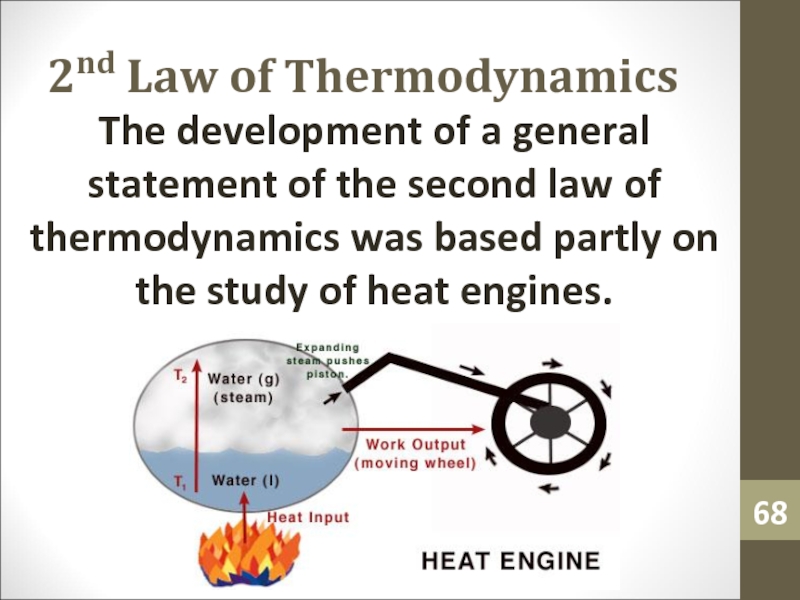
- 68. 2nd Law of ThermodynamicsThe development of a
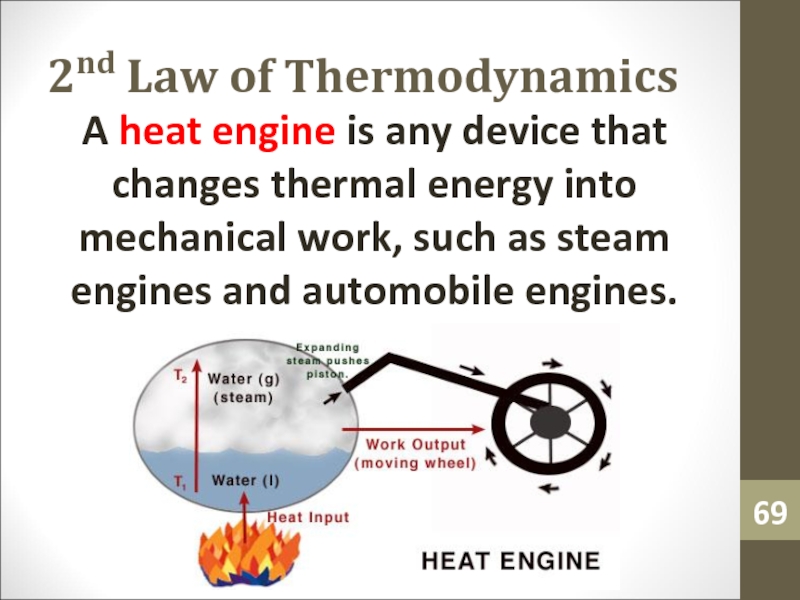
- 69. 2nd Law of ThermodynamicsA heat engine is
- 70. Скачать презентанцию
Learning Objectivesunderstand and apply first law of thermodynamicsdistinguish graphs of adiabatic and isothermal processesunderstand second law of thermodynamics
Слайды и текст этой презентации
Слайд 2Learning Objectives
understand and apply first law of thermodynamics
distinguish graphs of
adiabatic and isothermal processes
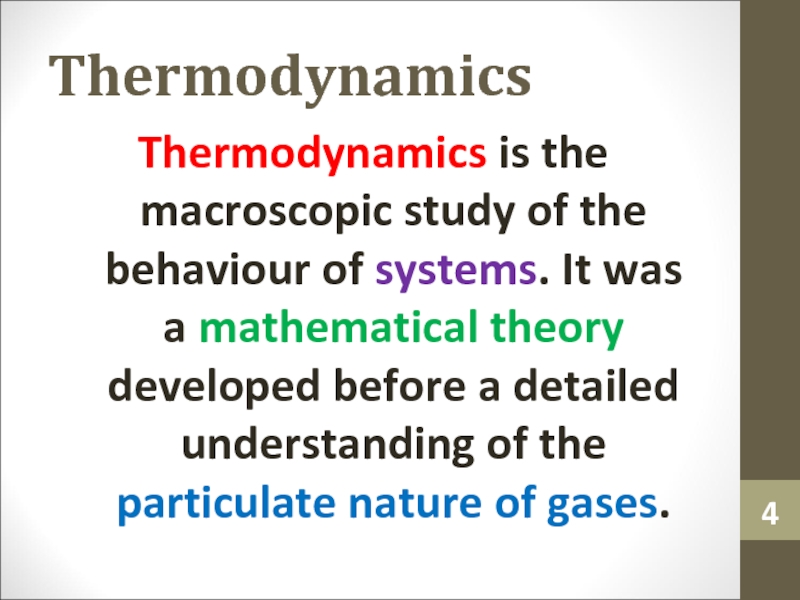
Слайд 4Thermodynamics
Thermodynamics is the macroscopic study of the behaviour of systems.
It was a mathematical theory developed before a detailed understanding
of the particulate nature of gases.Слайд 5Thermodynamic System
Thermodynamic System is a macroscopic aspect of a problem
than can be considered as a separate whole. An ideal
gas for example can have energy flowing in or out of it fromthe surroundings.
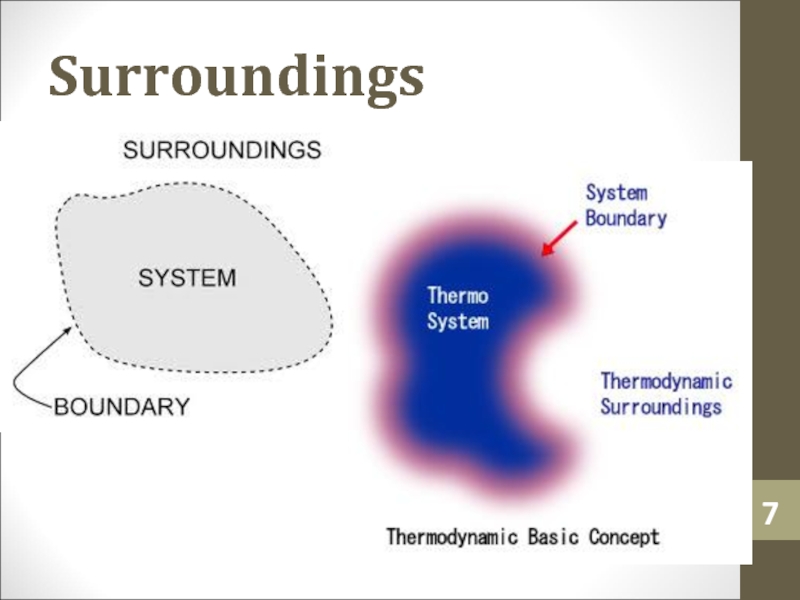
Слайд 6Surroundings
Surroundings is everything in the problem outside the
thermodynamic system.
Heat can flow from the system to the surroundings and
vice versa.Слайд 12Heat
Heat (Q) is an amount of thermal energy transferred from
the surroundings to an ideal gas. It is a result
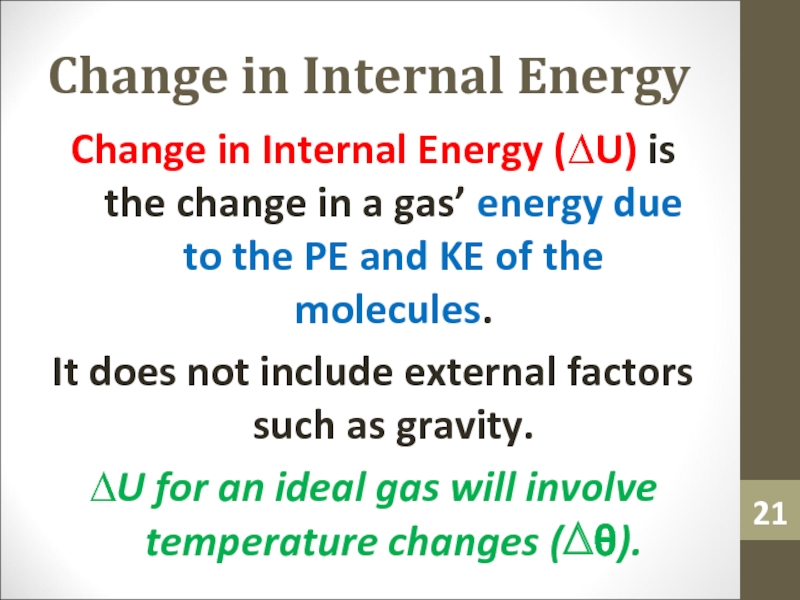
of a temperature difference.Слайд 21Change in Internal Energy
Change in Internal Energy (∆U) is the
change in a gas’ energy due to the PE and
KE of the molecules.It does not include external factors such as gravity.
∆U for an ideal gas will involve temperature changes (∆θ).
Слайд 22Change in Internal Energy
The change in internal energy (ΔU) of
a closed system will be equal to the energy added
to the system by heating minus the work done by the system on the surroundings.ΔU = Q – W
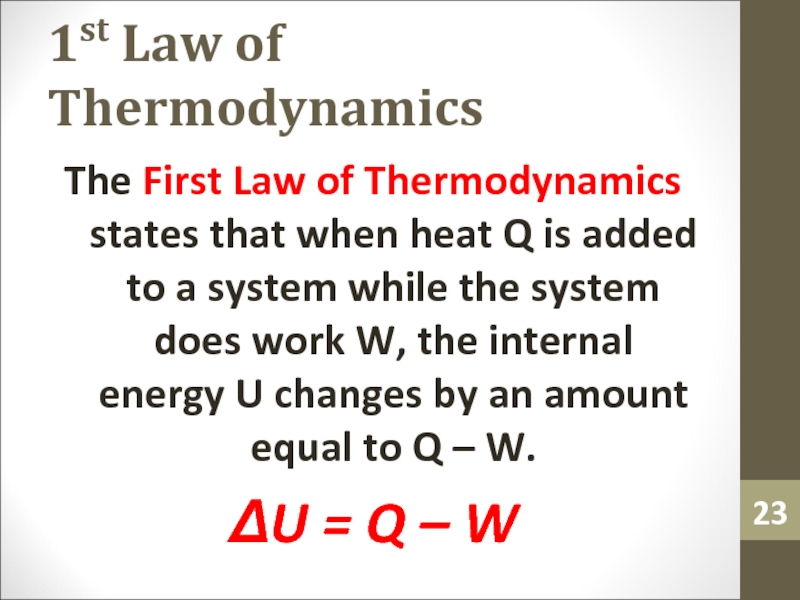
Слайд 231st Law of Thermodynamics
The First Law of Thermodynamics states that
when heat Q is added to a system while the
system does work W, the internal energy U changes by an amount equal to Q – W.ΔU = Q – W
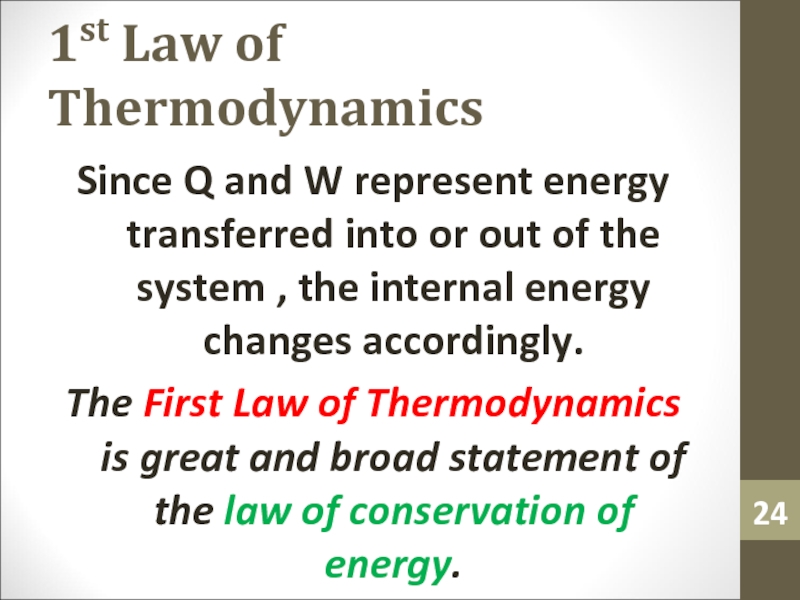
Слайд 241st Law of Thermodynamics
Since Q and W represent energy transferred
into or out of the system , the internal energy
changes accordingly.The First Law of Thermodynamics is great and broad statement of the law of conservation of energy.
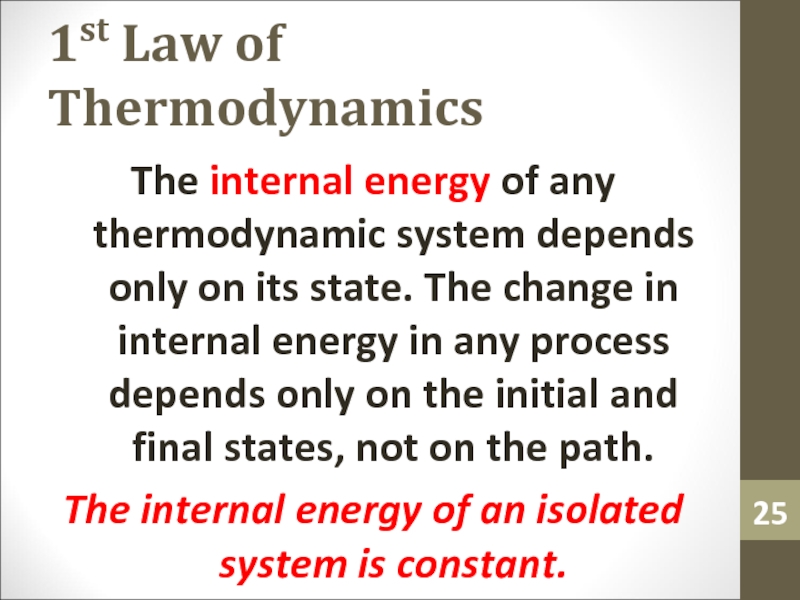
Слайд 251st Law of Thermodynamics
The internal energy of any thermodynamic system
depends only on its state. The change in internal energy
in any process depends only on the initial and final states, not on the path.The internal energy of an isolated system is constant.
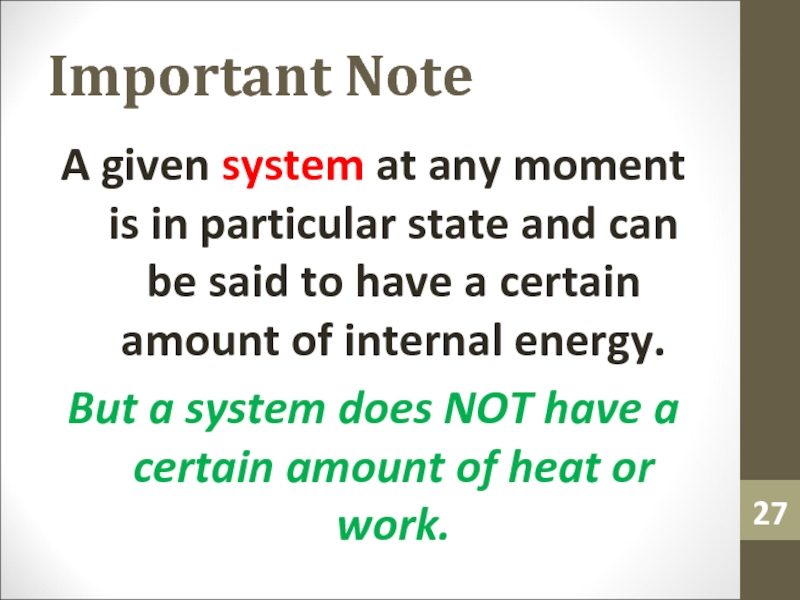
Слайд 27Important Note
A given system at any moment is in particular
state and can be said to have a certain amount
of internal energy.But a system does NOT have a certain amount of heat or work.
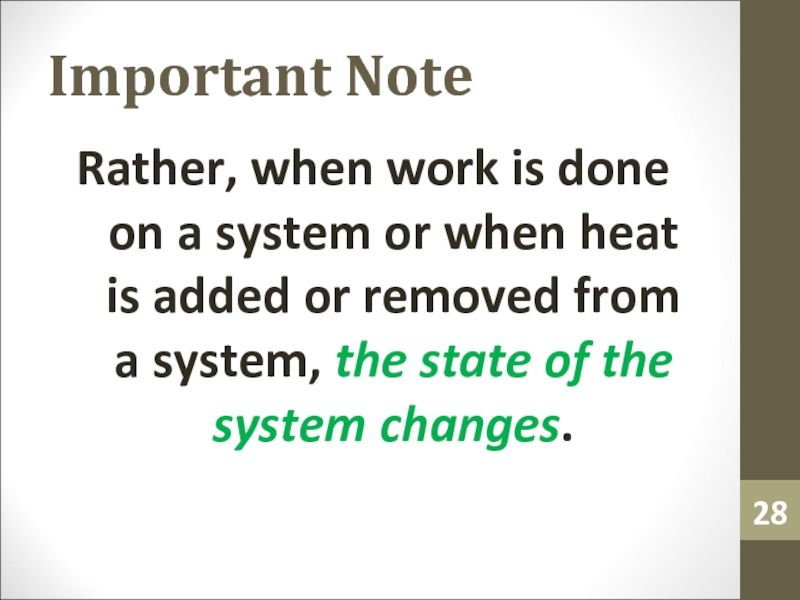
Слайд 28Important Note
Rather, when work is done on a system or
when heat is added or removed from a system, the
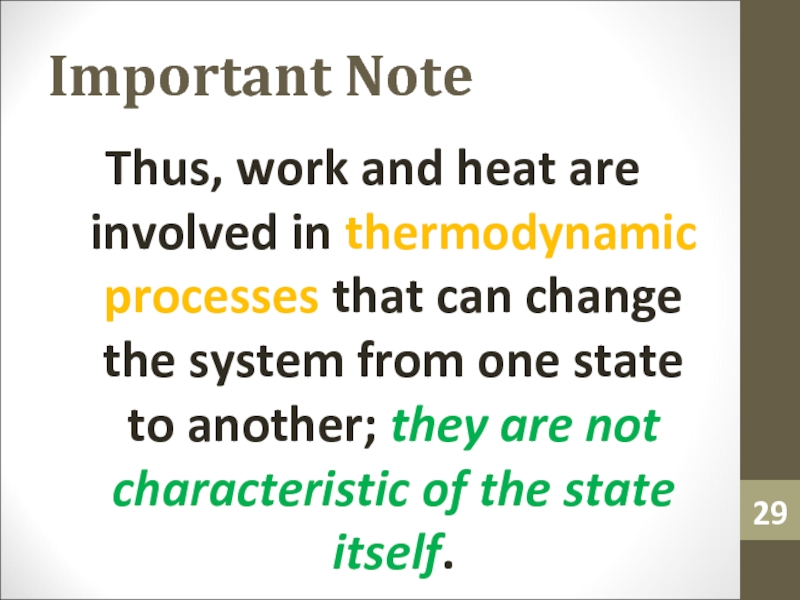
state of the system changes.Слайд 29Important Note
Thus, work and heat are involved in thermodynamic processes
that can change the system from one state to another;
they are not characteristic of the state itself.Слайд 30State Variables
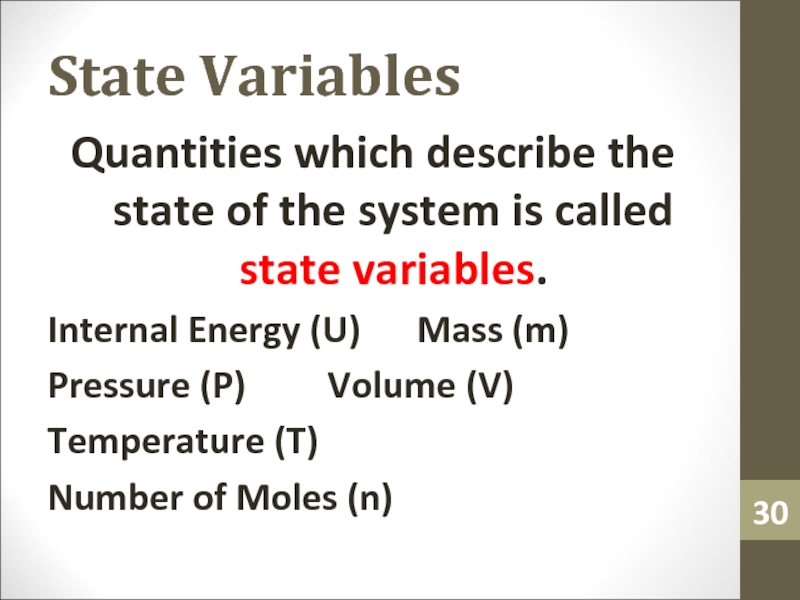
Quantities which describe the state of the system is
called state variables.
Internal Energy (U) Mass (m)
Pressure (P) Volume (V)
Temperature (T)
Number of Moles (n)
Слайд 31Sample Problem
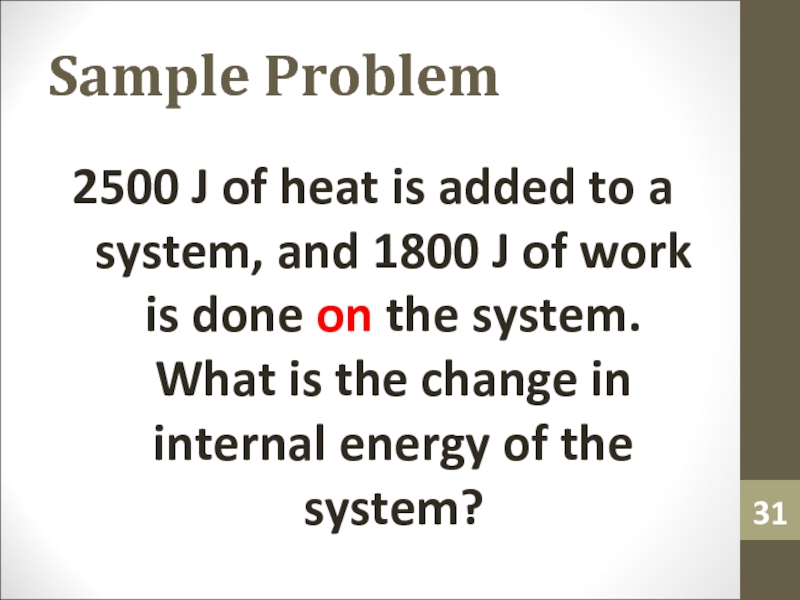
2500 J of heat is added to a system,
and 1800 J of work is done on the system.
What is the change in internal energy of the system?Слайд 32Sample Problem
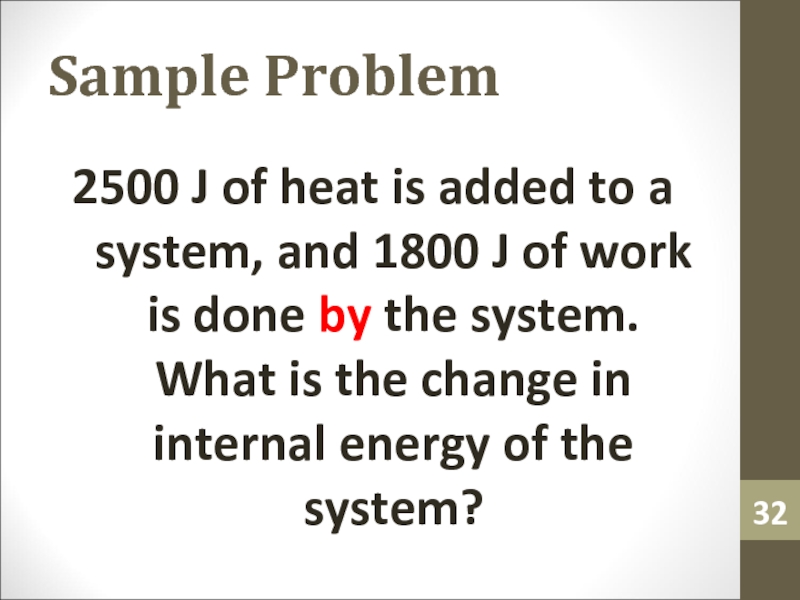
2500 J of heat is added to a system,
and 1800 J of work is done by the system.
What is the change in internal energy of the system?Слайд 33Isothermal Process and 1st Law of Thermodynamics
To analyze some thermodynamic
process in light of the 1st law of thermodynamics, consider
a fixed mass of an ideal gas enclosed in a container fitted with movable piston.Слайд 34Isothermal Process and 1st Law of Thermodynamics
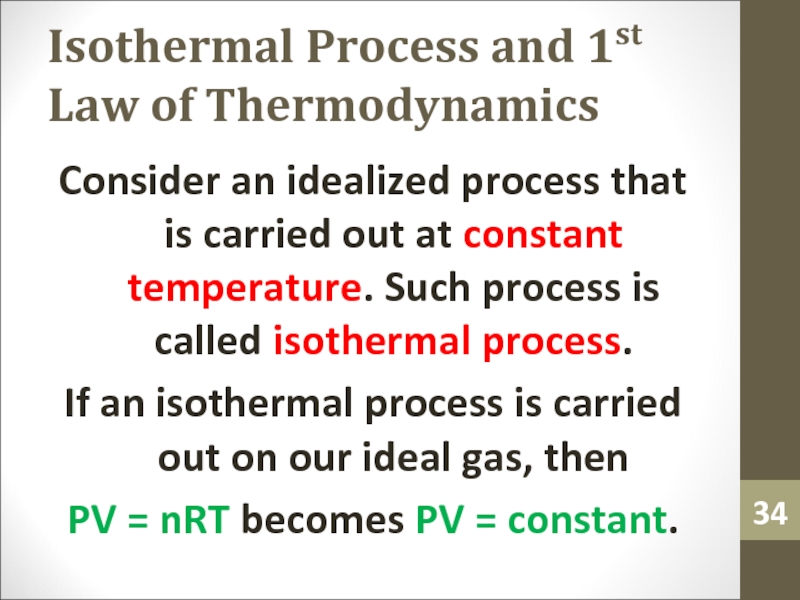
Consider an idealized process
that is carried out at constant temperature. Such process is
called isothermal process.If an isothermal process is carried out on our ideal gas, then
PV = nRT becomes PV = constant.
Слайд 35Isothermal Process and 1st Law of Thermodynamics
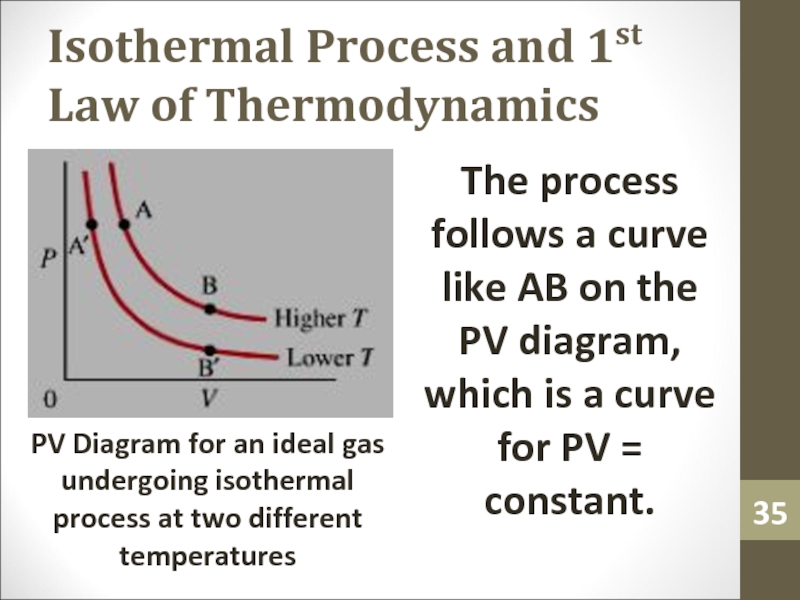
The process follows a
curve like AB on the PV diagram, which is a
curve for PV = constant.PV Diagram for an ideal gas undergoing isothermal process at two different temperatures
Слайд 36Isothermal Process and 1st Law of Thermodynamics
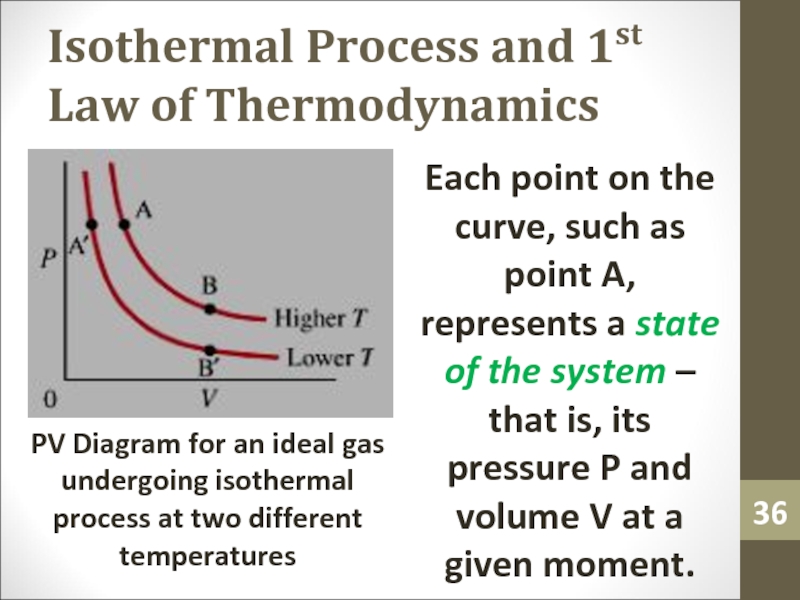
Each point on the
curve, such as point A, represents a state of the
system – that is, its pressure P and volume V at a given moment.PV Diagram for an ideal gas undergoing isothermal process at two different temperatures
Слайд 37Isothermal Process and 1st Law of Thermodynamics
The curves shown are
referred to as isotherms.
PV Diagram for an ideal gas undergoing
isothermal process at two different temperaturesСлайд 38Work Done
In the process A to D, the gas does
no work since the volume does not change
(isochoric).
Слайд 39Work Done
Going from D to B, the gas does work
equal to
PB(VB – VA).
This is the total work done in
the process ADB.(isobaric)
Слайд 40Work Done
If the pressure varies during a process, such as
for the isothermal process A,
W = PΔV
cannot be used directly
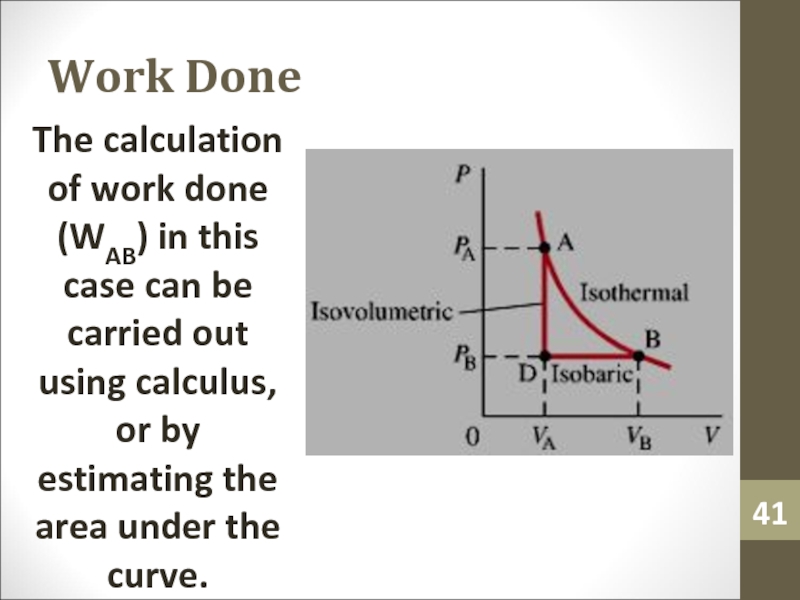
to determine the work.Слайд 41Work Done
The calculation of work done (WAB) in this case
can be carried out using calculus, or by estimating the
area under the curve.Слайд 42Adiabatic Process and 1st Law of Thermodynamics
An adiabatic process is
one in which no heat is allowed to flow into
or out of the system: Q = 0.This situation can occur if the system is extremely well insulated, or the process happens so quickly that heat – which flows slowly – has no time to flow in or out.
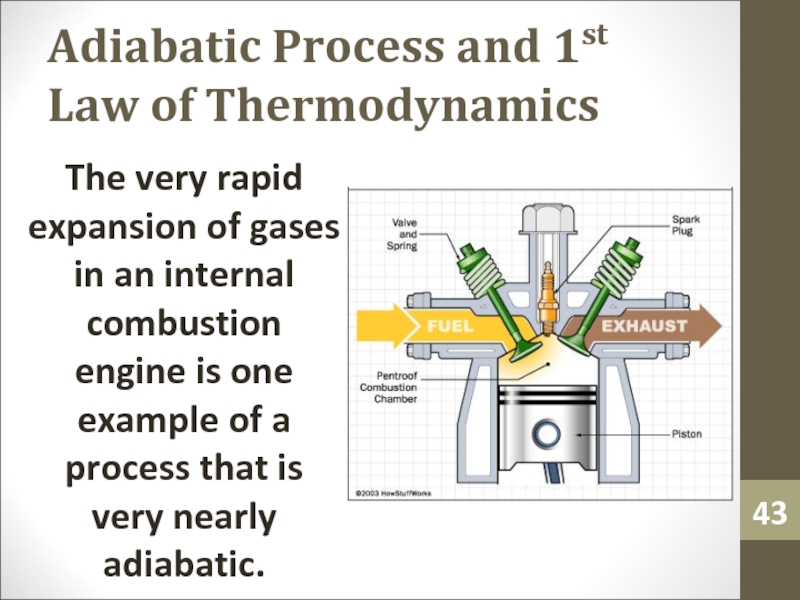
Слайд 43Adiabatic Process and 1st Law of Thermodynamics
The very rapid expansion
of gases in an internal combustion engine is one example
of a process that is very nearly adiabatic.Слайд 44Adiabatic Process and 1st Law of Thermodynamics
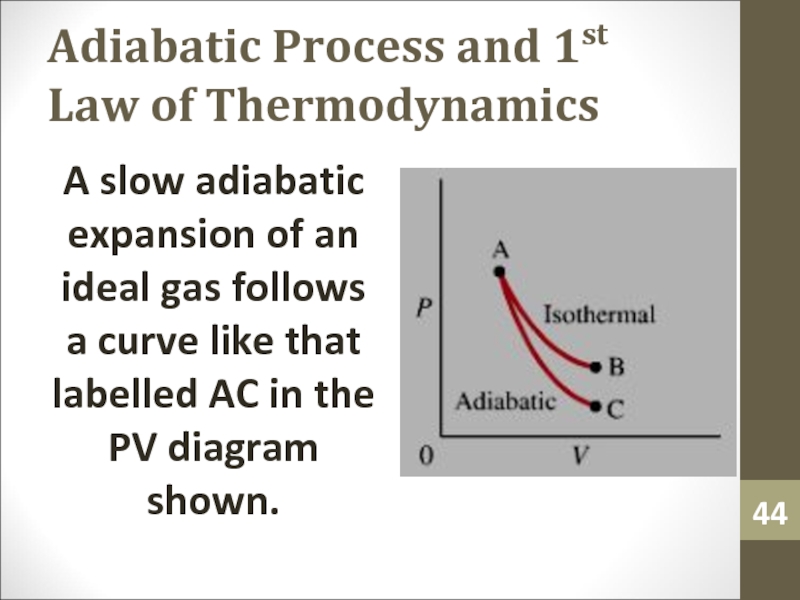
A slow adiabatic expansion
of an ideal gas follows a curve like that labelled
AC in the PV diagram shown.Слайд 45Adiabatic Process and 1st Law of Thermodynamics
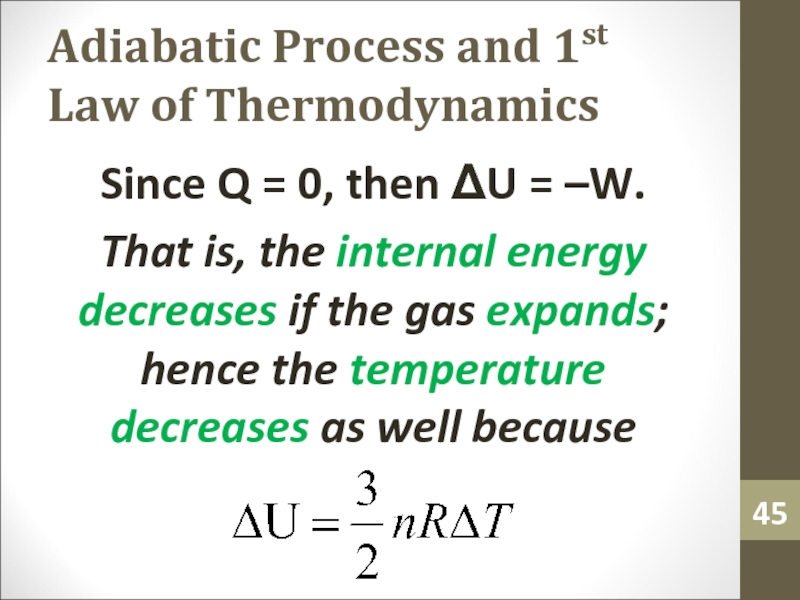
Since Q = 0,
then ΔU = –W.
That is, the internal energy decreases if
the gas expands; hence the temperature decreases as well becauseСлайд 46Adiabatic Process and 1st Law of Thermodynamics
In the reverse operation,
an adiabatic compression, work is done on the gas, and
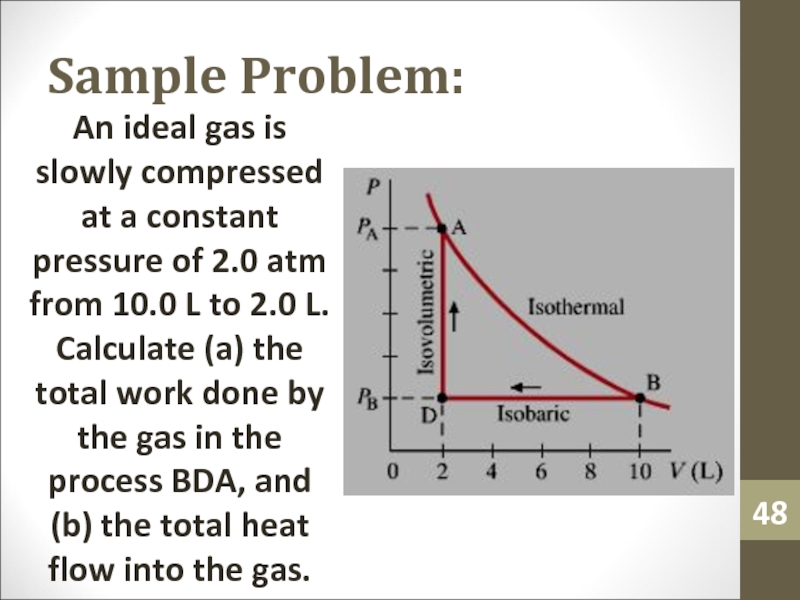
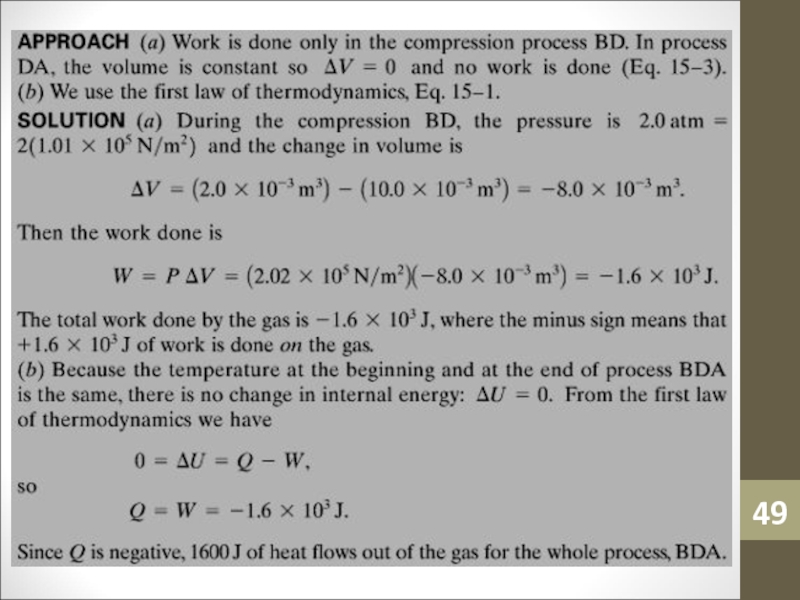
hence the internal energy increases and the temperature rises.Слайд 48Sample Problem:
An ideal gas is slowly compressed at a constant
pressure of 2.0 atm from 10.0 L to 2.0 L.
Calculate (a) the total work done by the gas in the process BDA, and (b) the total heat flow into the gas.Слайд 50Sample Problem:
In an engine, 0.25 moles of an ideal monatomic
gas in the cylinder expands rapidly and adiabatically against the
piston. In the process, the temperature of the gas drops from 1150 K to 400 K. How much work does the gas do?Слайд 52Sample Problem:
A gas in a container with a piston expands
isothermally. If thermal energy Q = 105 is given to
the gas, what is the work done by the gas?Слайд 56Sample Problem:
A monatomic gas is kept at constant pressure 3.00
× 106 Pa, initial volume 0.100 m3 and temperature 300
K. If the gas is compressed at constant pressure down a volume of 0.800 m3, find:(a) the work done on the gas; (b) the thermal energy taken out of the gas.
Слайд 582nd Law of Thermodynamics
There are many processes in thermodynamics that
are consistent with the first law but are nonetheless impossible.
Слайд 592nd Law of Thermodynamics
If you put a layer of salt
in a jar and cover it with a layer of
similar-sized grains of pepper, when you shake it you get a thorough mixture. But no matter how long you shake it, the mixture does not separate into layers again.Слайд 602nd Law of Thermodynamics
Coffee cups and glasses break spontaneously if
you drop them. But they don’t go back together spontaneously.
The
spontaneous (without the action of another agent) transfer of thermal energy from a cold body to hotter bodyСлайд 612nd Law of Thermodynamics
The air in a room suddenly occupying
just one half of the room and leaving the other
half empty.A glass of water at room temperature suddenly freezing, causing the temperature of the room to rise