Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Nuclear Structure. Some Properties of Nuclei.
Содержание
- 1. Nuclear Structure. Some Properties of Nuclei.
- 2. Some Properties of NucleiWe describe the atomic
- 3. Some Properties of NucleiCharge and MassThe atomic
- 4. Some Properties of NucleiIt is often convenient
- 5. Some Properties of NucleiThe Size and Structure
- 6. Some Properties of NucleiThe Size and Structure
- 7. Nuclear StabilitySome Properties of Nuclei(a) Potential energy
- 8. Nuclear StabilitySome Properties of NucleiNeutron number N
- 9. Nuclear Binding Energythe total mass of a
- 10. Nuclear Binding Energy
- 11. Nuclear ModelsThe Liquid-Drop ModelIn the liquid-drop model,
- 12. Nuclear ModelsThe Liquid-Drop ModelThe binding-energy curve plotted
- 13. Nuclear ModelsThe Shell ModelThe difference between measured
- 14. Nuclear ModelsThe Shell ModelA square potential well
- 15. RadioactivityThe process of spontaneous emission of radiation
- 16. Radioactivitywhere the constant N0 represents the number
- 17. RadioactivityThe half-life of a radioactive substance is
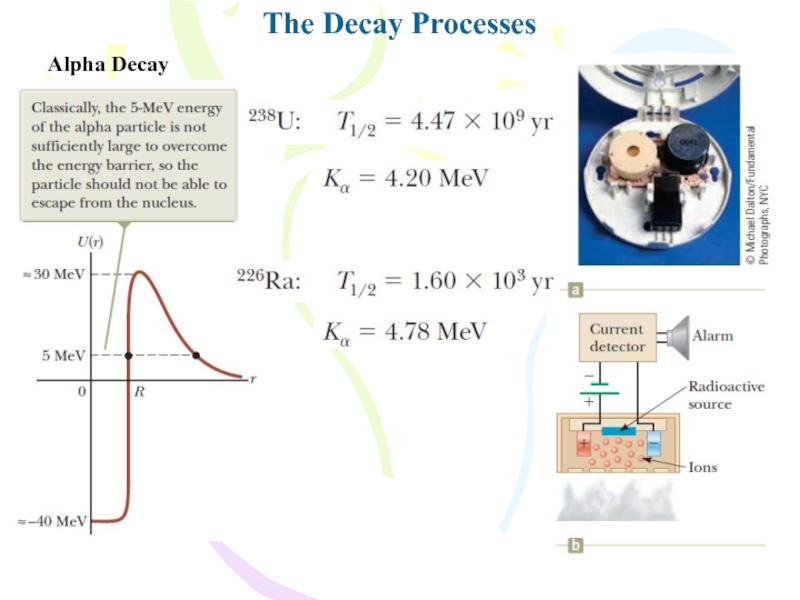
- 18. The Decay ProcessesAlpha Decaywhere X is called
- 19. The Decay ProcessesAlpha DecayThe alpha decay of
- 20. The Decay ProcessesAlpha Decay
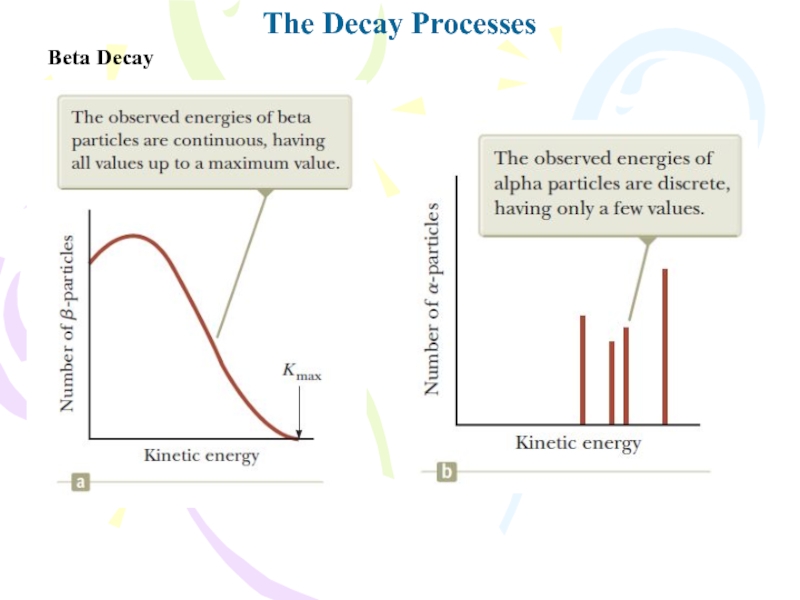
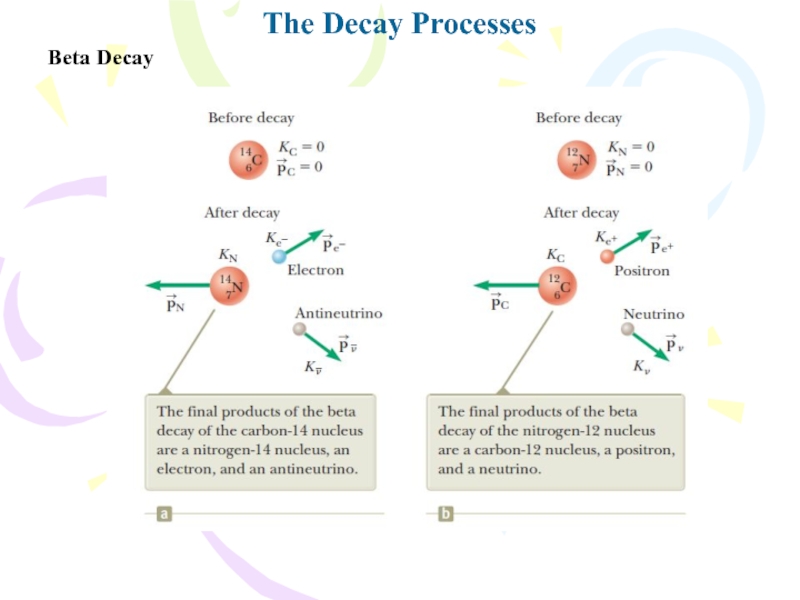
- 21. The Decay ProcessesBeta DecayTwo typical beta-decay processes are
- 22. The Decay ProcessesBeta Decay
- 23. The Decay ProcessesBeta Decay• It has zero
- 24. The Decay ProcessesBeta Decay
- 25. The Decay ProcessesBeta Decay
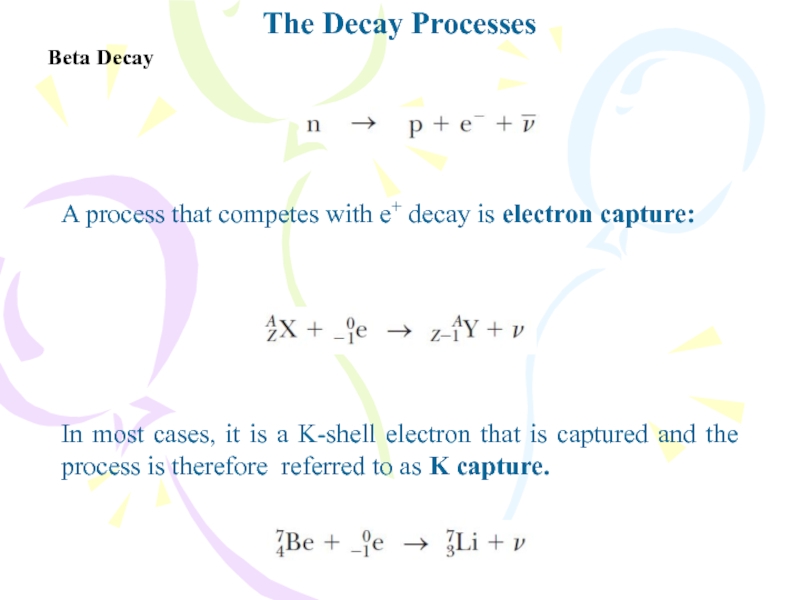
- 26. The Decay ProcessesBeta DecayA process that competes
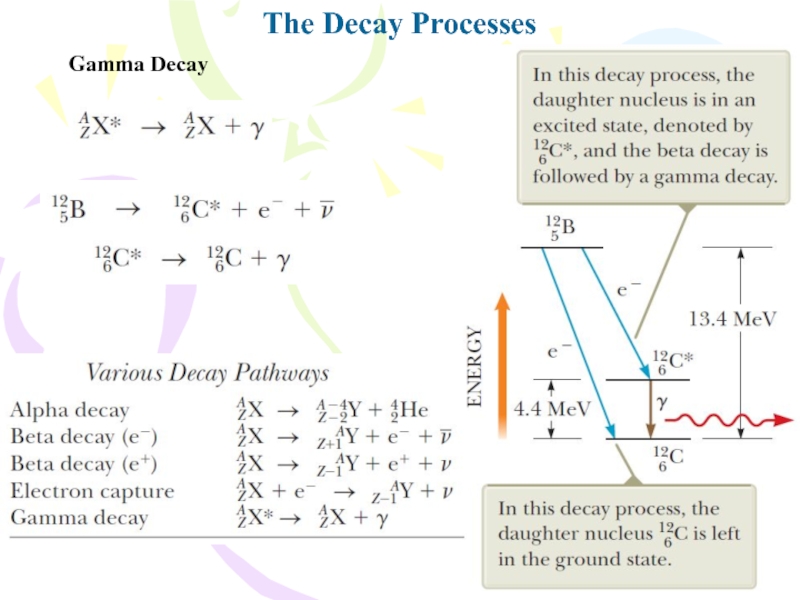
- 27. The Decay ProcessesGamma Decay
- 28. Natural RadioactivityRadioactive nuclei are generally classified into
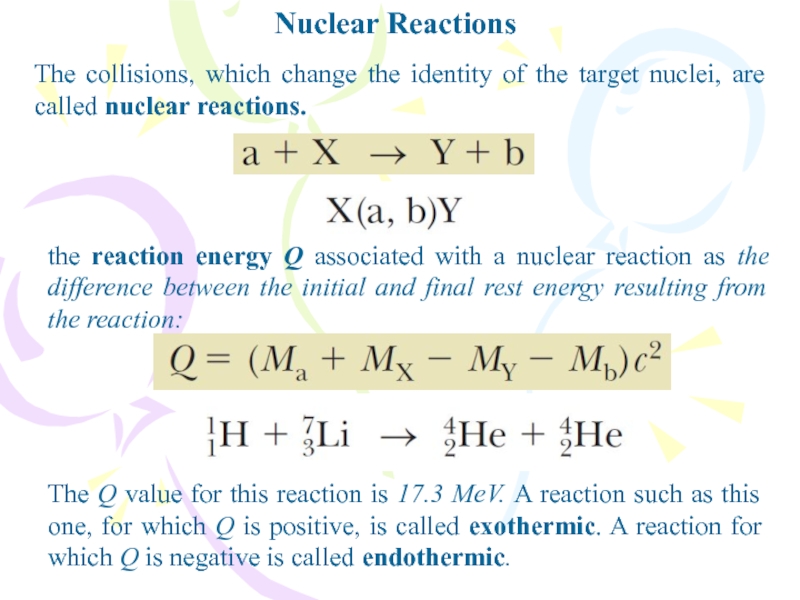
- 29. Nuclear ReactionsThe collisions, which change the identity
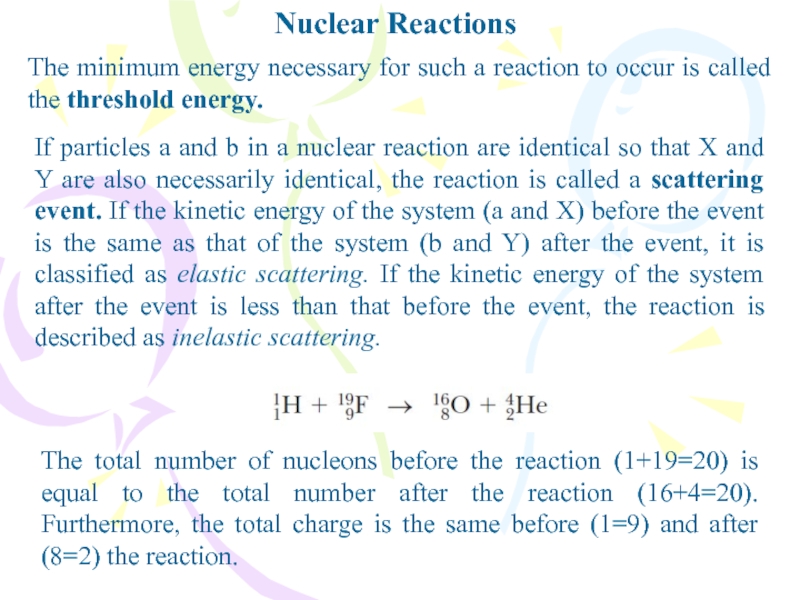
- 30. Nuclear ReactionsThe minimum energy necessary for such
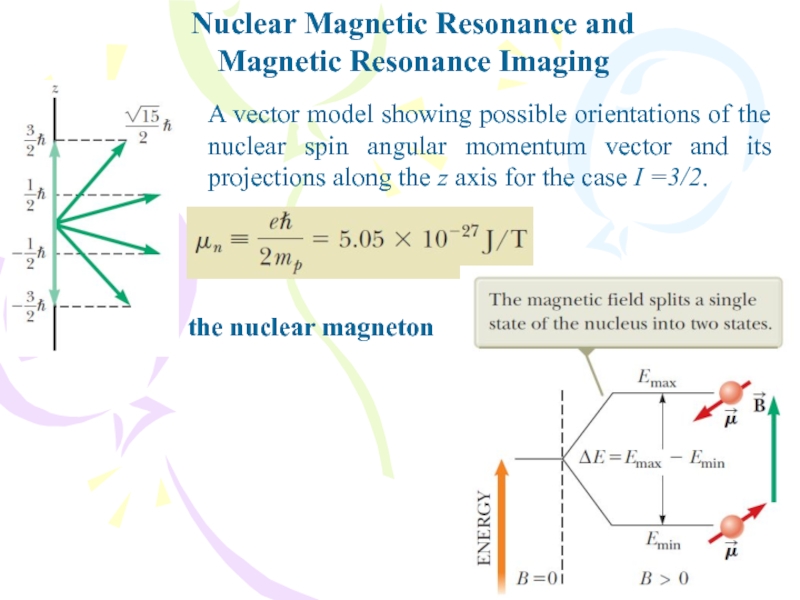
- 31. Nuclear Magnetic Resonance andMagnetic Resonance ImagingA vector
- 32. Nuclear Magnetic Resonance andMagnetic Resonance ImagingExperimental arrangement
- 33. Скачать презентанцию
Слайды и текст этой презентации
Слайд 1Course of lectures «Contemporary Physics: Part2»
Lecture №13
Nuclear Structure. Some Properties
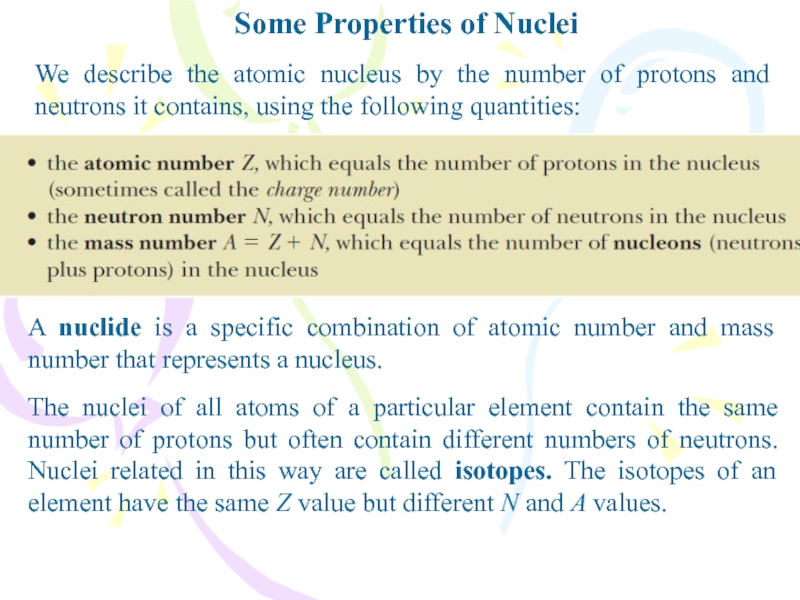
Слайд 2Some Properties of Nuclei
We describe the atomic nucleus by the
number of protons and neutrons it contains, using the following
quantities:A nuclide is a specific combination of atomic number and mass number that represents a nucleus.
The nuclei of all atoms of a particular element contain the same number of protons but often contain different numbers of neutrons. Nuclei related in this way are called isotopes. The isotopes of an element have the same Z value but different N and A values.
Слайд 3Some Properties of Nuclei
Charge and Mass
The atomic mass unit u
is defined in such a way that the mass of
one atom of the isotope 12C is exactly 12 u, where 1 u is equal to 1.660 539x10-27 kg.Слайд 4Some Properties of Nuclei
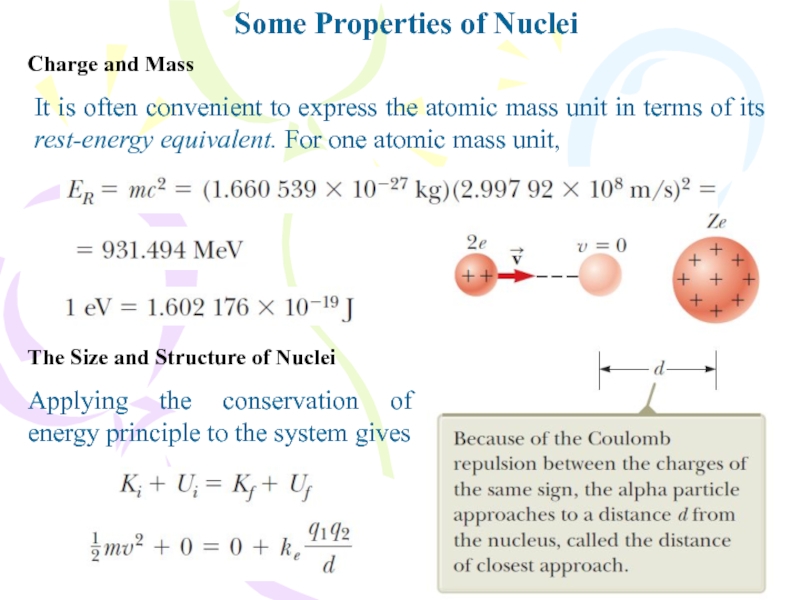
It is often convenient to express the
atomic mass unit in terms of its rest-energy equivalent. For
one atomic mass unit,Charge and Mass
The Size and Structure of Nuclei
Applying the conservation of energy principle to the system gives
Слайд 5Some Properties of Nuclei
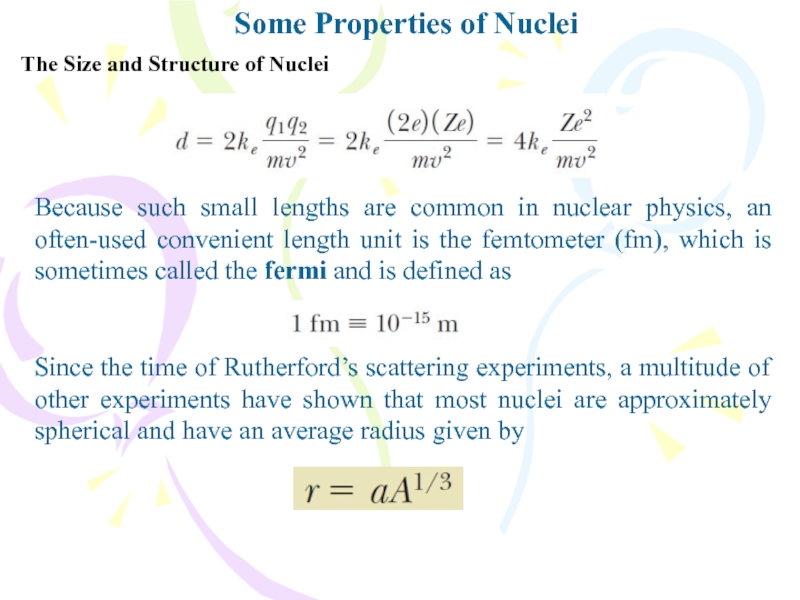
The Size and Structure of Nuclei
Because such
small lengths are common in nuclear physics, an often-used convenient
length unit is the femtometer (fm), which is sometimes called the fermi and is defined asSince the time of Rutherford’s scattering experiments, a multitude of other experiments have shown that most nuclei are approximately spherical and have an average radius given by
Слайд 6Some Properties of Nuclei
The Size and Structure of Nuclei
Because the
volume of a sphere is proportional to the cube of
its radius, the volume of a nucleus (assumed to be spherical) is directly proportional to A, the total number of nucleons. This proportionality suggests that all nuclei have nearly the same density. When nucleons combine to form a nucleus, they combine as though they were tightly packed spheres.A nucleus can be modeled as a cluster of tightly packed spheres, where each sphere is a nucleon.
Слайд 7Nuclear Stability
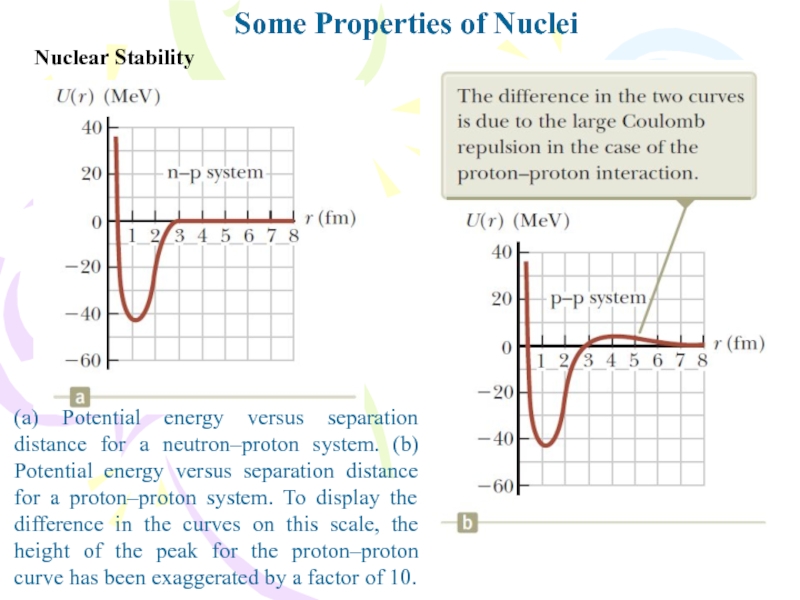
Some Properties of Nuclei
(a) Potential energy versus separation distance
for a neutron–proton system. (b) Potential energy versus separation distance
for a proton–proton system. To display the difference in the curves on this scale, the height of the peak for the proton–proton curve has been exaggerated by a factor of 10.Слайд 8Nuclear Stability
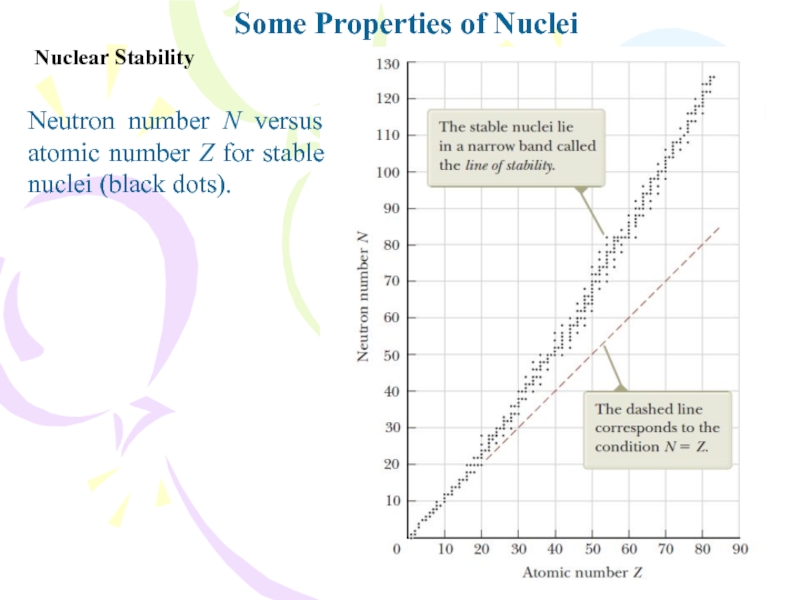
Some Properties of Nuclei
Neutron number N versus atomic number
Z for stable nuclei (black dots).
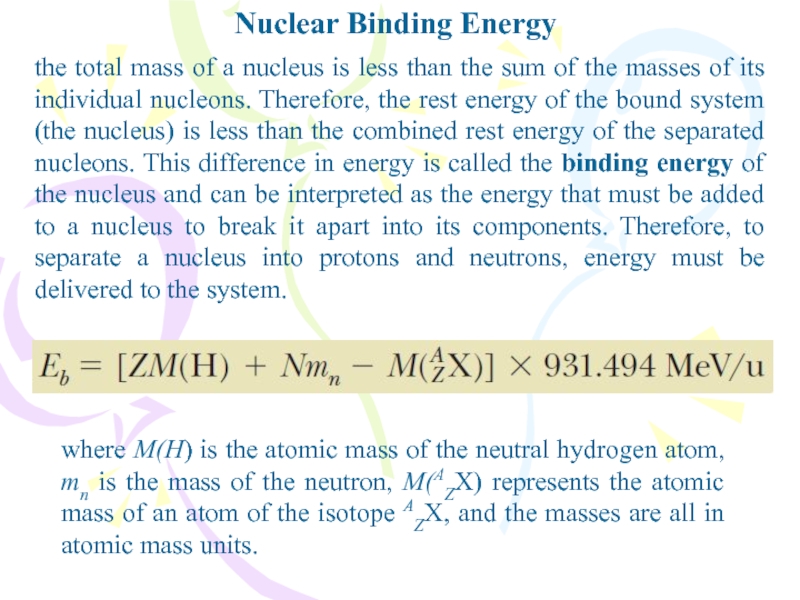
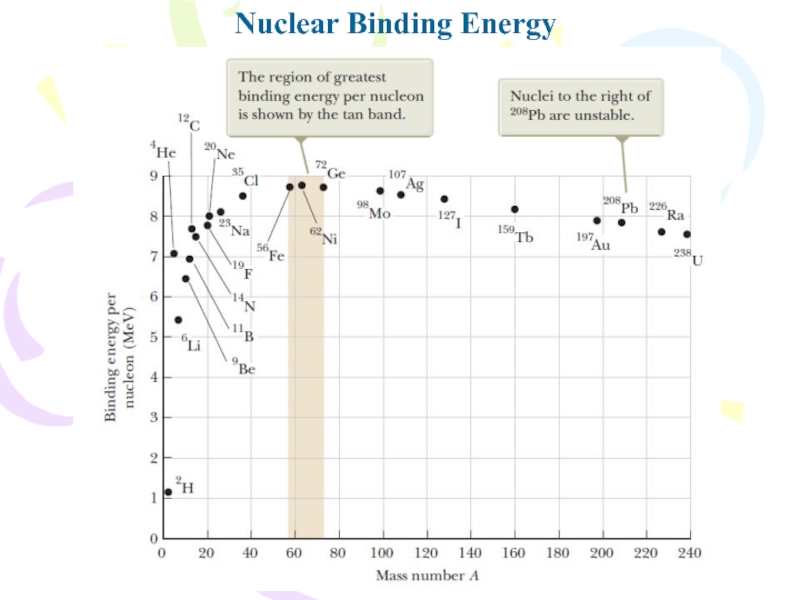
Слайд 9Nuclear Binding Energy
the total mass of a nucleus is less
than the sum of the masses of its individual nucleons.
Therefore, the rest energy of the bound system (the nucleus) is less than the combined rest energy of the separated nucleons. This difference in energy is called the binding energy of the nucleus and can be interpreted as the energy that must be added to a nucleus to break it apart into its components. Therefore, to separate a nucleus into protons and neutrons, energy must be delivered to the system.where M(H) is the atomic mass of the neutral hydrogen atom, mn is the mass of the neutron, M(AZX) represents the atomic mass of an atom of the isotope AZX, and the masses are all in atomic mass units.
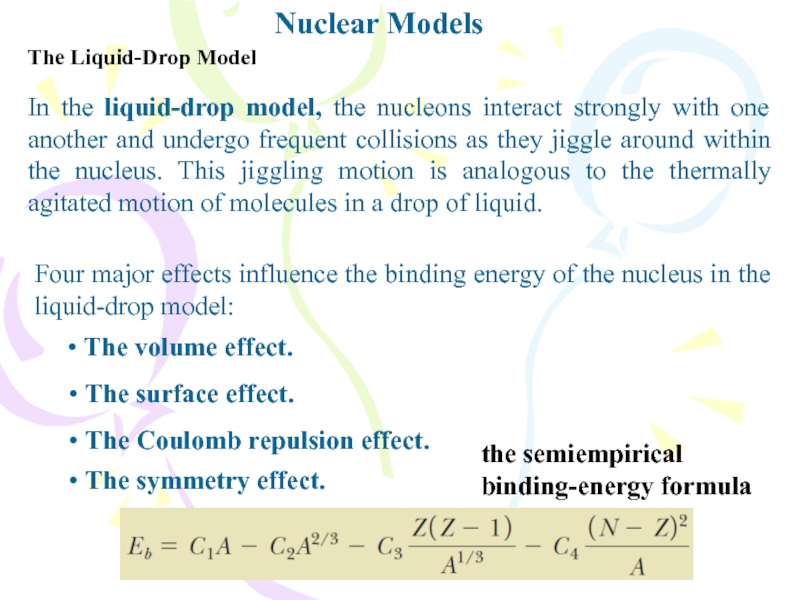
Слайд 11Nuclear Models
The Liquid-Drop Model
In the liquid-drop model, the nucleons interact
strongly with one another and undergo frequent collisions as they
jiggle around within the nucleus. This jiggling motion is analogous to the thermally agitated motion of molecules in a drop of liquid.Four major effects influence the binding energy of the nucleus in the liquid-drop model:
• The volume effect.
• The surface effect.
• The Coulomb repulsion effect.
• The symmetry effect.
the semiempirical binding-energy formula
Слайд 12Nuclear Models
The Liquid-Drop Model
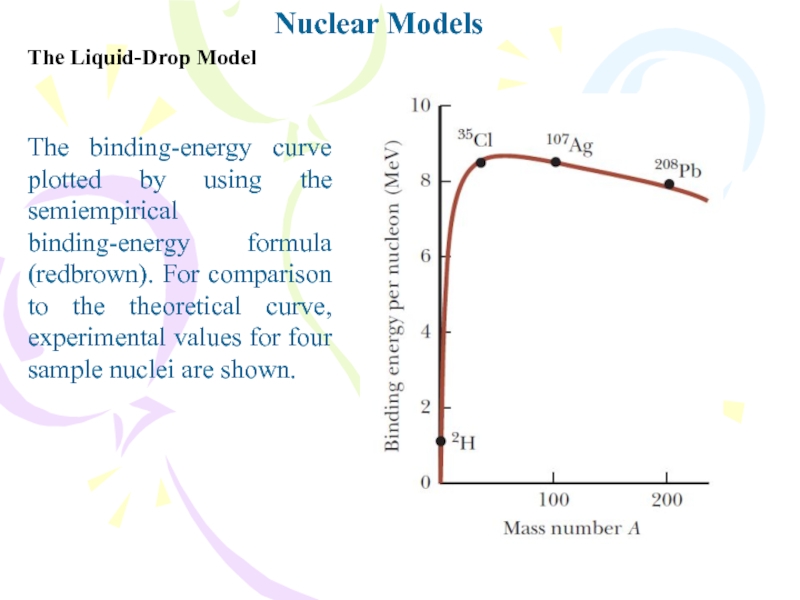
The binding-energy curve plotted by using the
semiempirical binding-energy formula (redbrown). For comparison to the theoretical curve,
experimental values for four sample nuclei are shown.Слайд 13Nuclear Models
The Shell Model
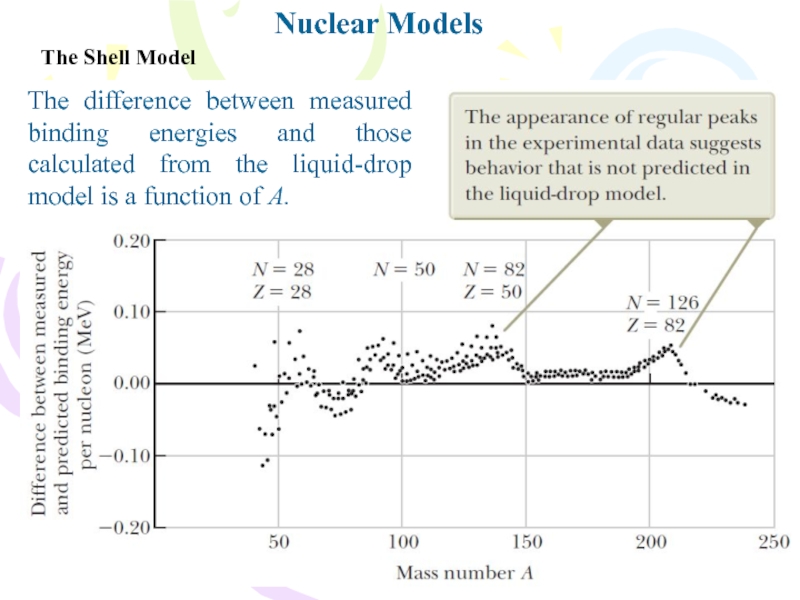
The difference between measured binding energies and
those calculated from the liquid-drop model is a function of
A.Слайд 14Nuclear Models
The Shell Model
A square potential well containing 12 nucleons.
The red spheres represent protons, and the gray spheres represent
neutrons.Слайд 15Radioactivity
The process of spontaneous emission of radiation is called radioactivity.
where
λ, called the decay constant, is the probability of decay
per nucleus per second.Слайд 16Radioactivity
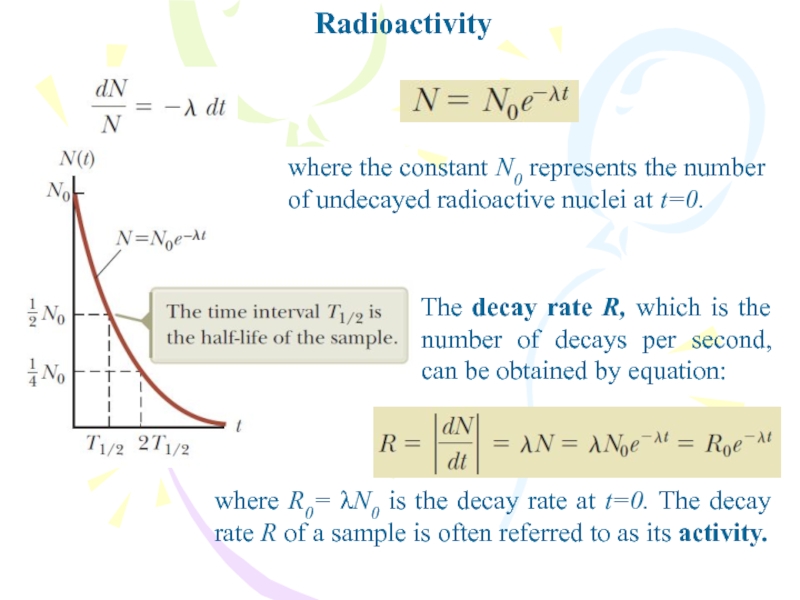
where the constant N0 represents the number of undecayed radioactive
nuclei at t=0.
The decay rate R, which is the number
of decays per second, can be obtained by equation:where R0= λN0 is the decay rate at t=0. The decay rate R of a sample is often referred to as its activity.
Слайд 17Radioactivity
The half-life of a radioactive substance is the time interval
during which half of a given number of radioactive nuclei
decay.A frequently used unit of activity is the curie (Ci), defined as
This value was originally selected because it is the approximate activity of 1 g of radium. The SI unit of activity is the becquerel (Bq):
Слайд 18The Decay Processes
Alpha Decay
where X is called the parent nucleus
and Y the daughter nucleus.
When the nucleus of one element
changes into the nucleus of another as happens in alpha decay, the process is called spontaneous decay.the disintegration energy of the system
Слайд 19The Decay Processes
Alpha Decay
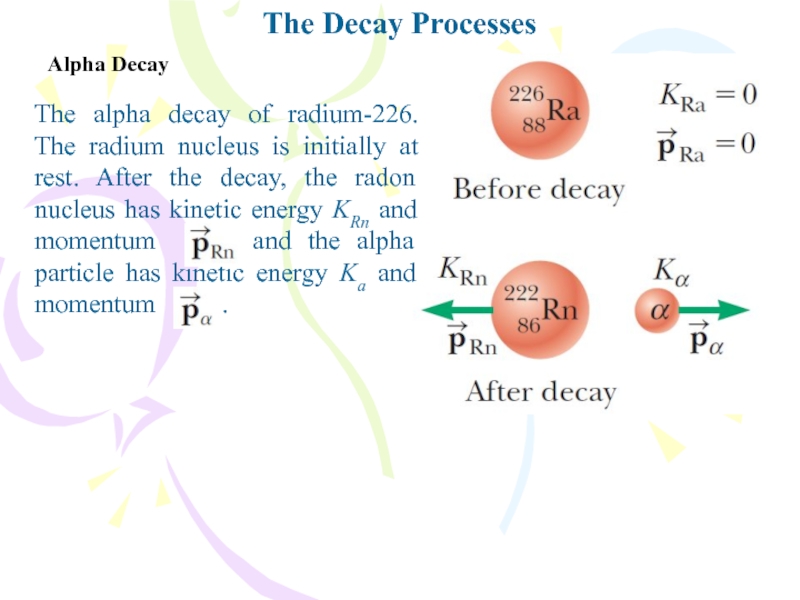
The alpha decay of radium-226. The radium
nucleus is initially at rest. After the decay, the radon
nucleus has kinetic energy KRn and momentum and the alpha particle has kinetic energy Ka and momentum .Слайд 23The Decay Processes
Beta Decay
• It has zero electric charge.
• Its
mass is either zero (in which case it travels at
the speed of light) or very small; much recent persuasive experimental evidence suggests that the neutrino mass is not zero. Current experiments place the upper bound of the mass of the neutrino at approximately 7 eV/c2.• It has a spin of 1/2, which allows the law of conservation of angular momentum to be satisfied in beta decay.
• It interacts very weakly with matter and is therefore very difficult to detect.
The neutrino has the following properties:
Слайд 26The Decay Processes
Beta Decay
A process that competes with e+ decay
is electron capture:
In most cases, it is a K-shell electron
that is captured and the process is therefore referred to as K capture.Слайд 28Natural Radioactivity
Radioactive nuclei are generally classified into two groups: (1)
unstable nuclei found in nature, which give rise to natural
radioactivity, and (2) unstable nuclei produced in the laboratory through nuclear reactions, which exhibit artificial radioactivity.Слайд 29Nuclear Reactions
The collisions, which change the identity of the target
nuclei, are called nuclear reactions.
the reaction energy Q associated with
a nuclear reaction as the difference between the initial and final rest energy resulting from the reaction:The Q value for this reaction is 17.3 MeV. A reaction such as this one, for which Q is positive, is called exothermic. A reaction for which Q is negative is called endothermic.
Слайд 30Nuclear Reactions
The minimum energy necessary for such a reaction to
occur is called the threshold energy.
If particles a and b
in a nuclear reaction are identical so that X and Y are also necessarily identical, the reaction is called a scattering event. If the kinetic energy of the system (a and X) before the event is the same as that of the system (b and Y) after the event, it is classified as elastic scattering. If the kinetic energy of the system after the event is less than that before the event, the reaction is described as inelastic scattering.The total number of nucleons before the reaction (1+19=20) is equal to the total number after the reaction (16+4=20). Furthermore, the total charge is the same before (1=9) and after (8=2) the reaction.
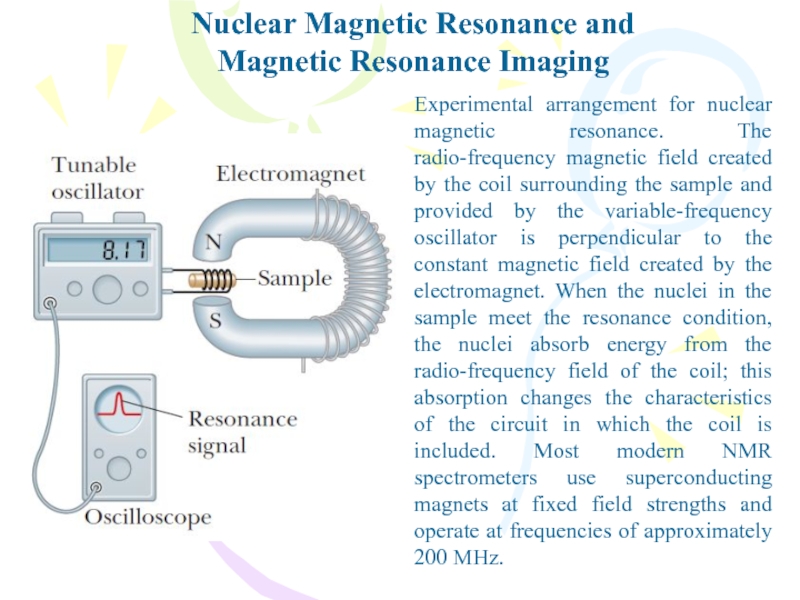
Слайд 31Nuclear Magnetic Resonance and
Magnetic Resonance Imaging
A vector model showing possible
orientations of the nuclear spin angular momentum vector and its
projections along the z axis for the case I =3/2.the nuclear magneton