Слайд 1EBOLA VIRUS DISEASE IN PREGNANCY: clinical histopathologic and immunohistochemical findings
Nurdilda Nargiz, Kani Aidana, Berekekyzy Zhanerke, Bagytzhanova Aina, Sagadinov Sagyndyk
Faculty
of Education and Humanites
Suleyman Demirel University, Kazakhstan
Слайд 2Thanks to ...
Atis Muehlenbachs, Olimpia de la Rosa Vázquez, Daniel
G. Bausch, Ilana J. Schafer, Christopher D. Paddock, Jean Paul
Nyakio, Papys Lame, Eric Bergeron, Andrea M. McCollum, Cynthia S. Goldsmith, Brigid C. Bollweg, Miriam Alía Prieto, Robert Shongo Lushima, Benoit Kebela Ilunga, Stuart T. Nichol, Wun-Ju Shieh, Ute Ströher, Pierre E. Rollin, Sherif R. Zaki
For such great article!
Слайд 3EBOLA VIRUS (EVD)
An infectious
Generally fetal disease marked by
fever
Severe internal
bleeding
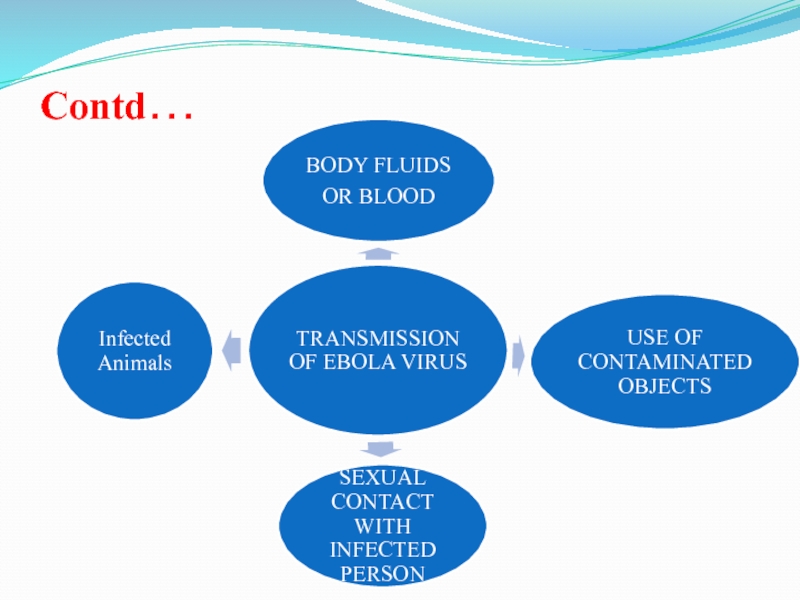
Spread throughout contacts with
Body fluids by Filovirus (Ebola Virus)
HOST
Unknown
Named because of Ebola River RIVER
Слайд 5FIRST APPEARANCE OF EVD
In Sudan and Zaire in 1976
FIRST OUTBREAK
In Sudan
Infected over 284 people
Killing 53% of victim
Another
strain appeared
Infected another 318 people
Mortality rate was 86%
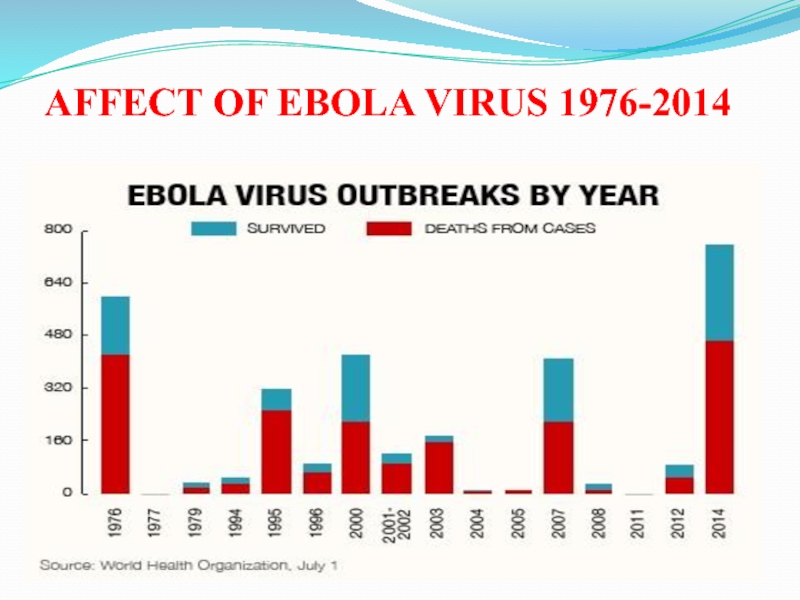
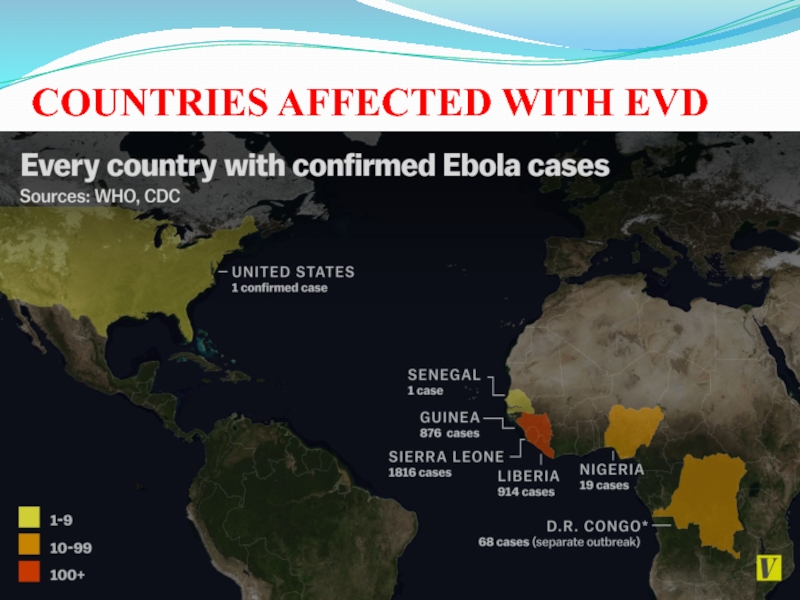
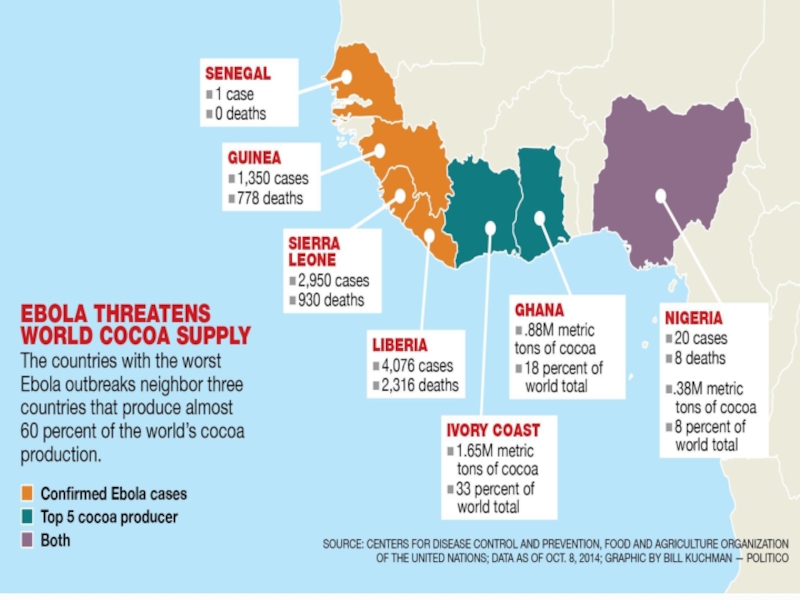
Слайд 6AFFECT OF EBOLA VIRUS 1976-2014
Слайд 7Species of Ebolaviruses
Ebolaviruses are closely related to species in the
genus Marburgvirus, which was discovered in 1967, and the two
are the only members of the Filoviridae that cause epidemic human disease. Five species of ebolaviruses—known as Zaire ebolavirus, Sudan ebolavirus, Taï Forest ebolavirus, Reston ebolavirus, and Bundibugyo ebolavirus, named for their outbreak locations—have been described. The viruses are known commonly as Ebola virus (EBOV), Sudan virus (SUDV), Taï Forest virus (TAFV), Reston virus (RESTV), and Bundibugyo virus (BDBV).
Слайд 8Ebola virus disease (EVD) and Marburg virus disease are caused
by viruses of the Ebolavirus and Marburgvirus genera (family Filoviridae).
Here, we collectively refer to Ebola virus (EBOV), Sudan virus (SUDV) and Bundibugyo virus (BDBV) all within the Ebolavirus genus as ebolaviruses. Filovirus infection during pregnancy is associated with maternal hemorrhage, preterm labor, miscarriage, and maternal and neonatal death. Supplementary Table 1 presents a summary of the scientific literature to date; maternal death occurred in 102 of 125 reported cases (82%), and there was uniform loss of offspring, whether by miscarriage, stillbirth, or neonatal death. Of the 18 live births, the longest survival was 19 days.
Слайд 9Despite the severity of filovirus infection in pregnancy for both
mother and child, very little is known regarding pathogenesis. Fetal-placental
viral tropism has been hypothesized due to recent observations during the 2013–2016 West Africa EBOV outbreak: pregnant women were noted to survive EVD and clear virus from the blood without fetal loss during acute infection and to deliver stillbirths in the subsequent weeks and months with relatively high EBOV RNA levels in placental and fetal tissue swab specimens [7–10]. We report clinical, histopathologic, and immunohistochemical findings of SUDV and BDBV infections in 2 pregnant women and their offspring that help shed light on the pathogenesis of fetal infection and loss in EVD.
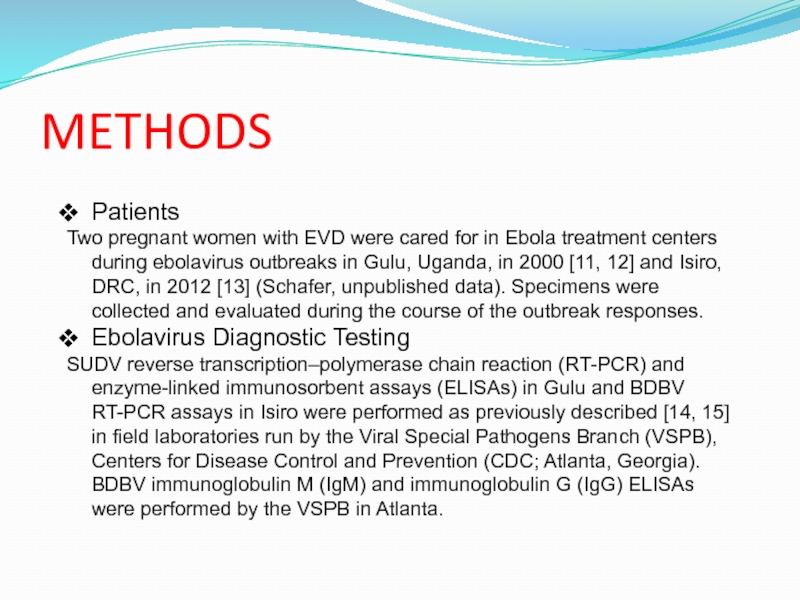
Слайд 10METHODS
Patients
Two pregnant women with EVD were cared for in Ebola
treatment centers during ebolavirus outbreaks in Gulu, Uganda, in 2000
[11, 12] and Isiro, DRC, in 2012 [13] (Schafer, unpublished data). Specimens were collected and evaluated during the course of the outbreak responses.
Ebolavirus Diagnostic Testing
SUDV reverse transcription–polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assays (ELISAs) in Gulu and BDBV RT-PCR assays in Isiro were performed as previously described [14, 15] in field laboratories run by the Viral Special Pathogens Branch (VSPB), Centers for Disease Control and Prevention (CDC; Atlanta, Georgia). BDBV immunoglobulin M (IgM) and immunoglobulin G (IgG) ELISAs were performed by the VSPB in Atlanta.
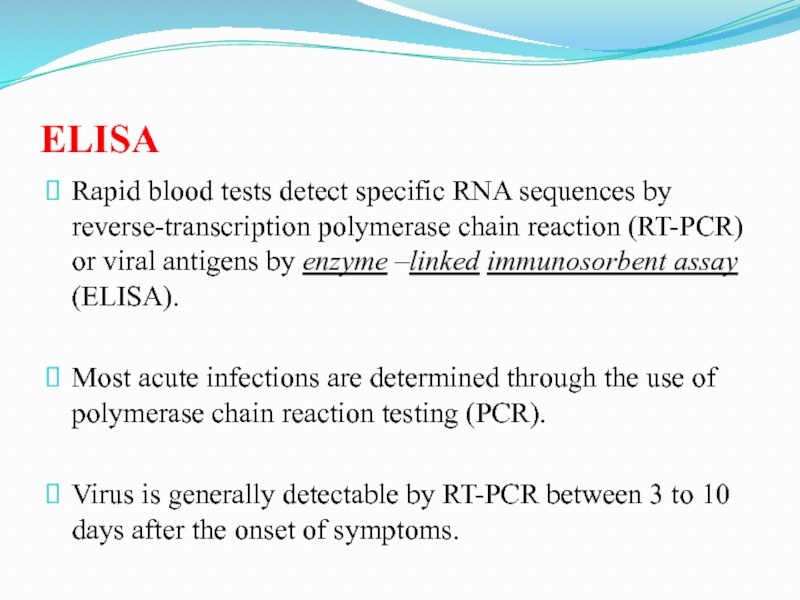
Слайд 11ELISA
Rapid blood tests detect specific RNA sequences by reverse-transcription polymerase
chain reaction (RT-PCR) or viral antigens by enzyme –linked immunosorbent
assay (ELISA).
Most acute infections are determined through the use of polymerase chain reaction testing (PCR).
Virus is generally detectable by RT-PCR between 3 to 10 days after the onset of symptoms.
Слайд 12Histopathologic Analysis, Immunohistochemical Analysis, and Transmission Electron Microscopy
Placenta (Gulu and
Isiro), fetal tissues (Gulu), and a postmortem skin biopsy (Isiro)
were collected and placed in 10% neutral buffered formalin and transported to the CDC, where the samples were processed using standard histological methods. The identification and scoring of malarial parasite pigment was performed as previously described [16]. Immunohistochemical analysis for ebolavirus antigens was performed using a polymer-based indirect immunoalkaline phosphatase detection system for colorimetric detection (Biocare Medical, Concord, California). Rabbit polyclonal antisera against EBOV, SUDV, and Reston virus and EBOV hyperimmune mouse ascitic fluid (courtesy of Thomas Ksiazek, VSPB, CDC), previously shown to detect SUDV and BDBV antigens, were each used at a 1:1000 dilution with appropriate positive and negative controls [17]. On-slide embedding and transmission electron microscopy was performed as previously described [18].
Слайд 13RESULTS. Patient 1
Patient 1, Gulu, Uganda, 30-year-old housewife
Symptoms: asthenia, anorexia,
abdominal pain, nausea, vomiting, diarrhea, and dry cough - presented
with a 1-day of illness. Has been pregnant for 28 weeks. Next day, she had temperature of 36.7°C, here heart rage was 120 beats/minute, and her respiratory rate was 24 breaths/minute, with an oxygen saturation level of 92% by pulse oximetry. Her blood tested positive for SUDV by both ELISA antigen assay and nested RT-PCR.
On day four of illness, the patient spontaneously delivered a dead but apparently morphologically normal fetus and placenta. The degree of vaginal bleeding did not seem abnormal for a stillbirth. Over the next 3 days, the patient complained of joint pain and swelling, throat and chest pain, persistent dry cough, dyspnea, and, briefly, hiccups. Her wrists and knees were visibly swollen and tender to the touch, and pulmonary rales persisted. She was consistently febrile. Disease severity peaked at day 7 of illness, when vital signs were an axillary temperature of 37.8°C, a heart rate of 128 beats/minute, a respiratory rate of 30 breaths per minute, and an oxygen saturation level of 90%. She gradually improved, and she was discharged on day 13 with normal vital signs and all symptoms resolved.
Слайд 14Pathologic Findings
The placenta had mild subchorionitis and a moderate amount
of malarial parasite pigment (hemozoin) in fibrin and within macrophages
embedded in fibrin (Figure 1A). No parasitized erythrocytes or malarial intervillous inflammatory infiltrates were present. By electron microscopy, hemozoin crystallites were identified (Figure 1B), but no ebolavirus virions were seen. The umbilical cord was normal.
Immunohistochemical analysis revealed ebolavirus antigen in the placenta, primarily within areas of fibrin deposition, localized to embedded maternal mononuclear cells, including malarial parasite pigment–laden macrophages (Figure 1C). Focal immunostaining was seen within the syncytiotrophoblast (Figure 1D). The decidua, fetal placental villous stroma, amnion, and umbilical cord were negative by immunohistochemical analysis, and no tissue necrosis or viral inclusions were noted.
Fetal tissues (lung, heart, liver, spleen, kidney, skin, and bone marrow) were well preserved with minimal autolysis, were normal for gestational age, and had no necrosis or viral inclusions. All fetal tissues were negative by immunohistochemical analysis.

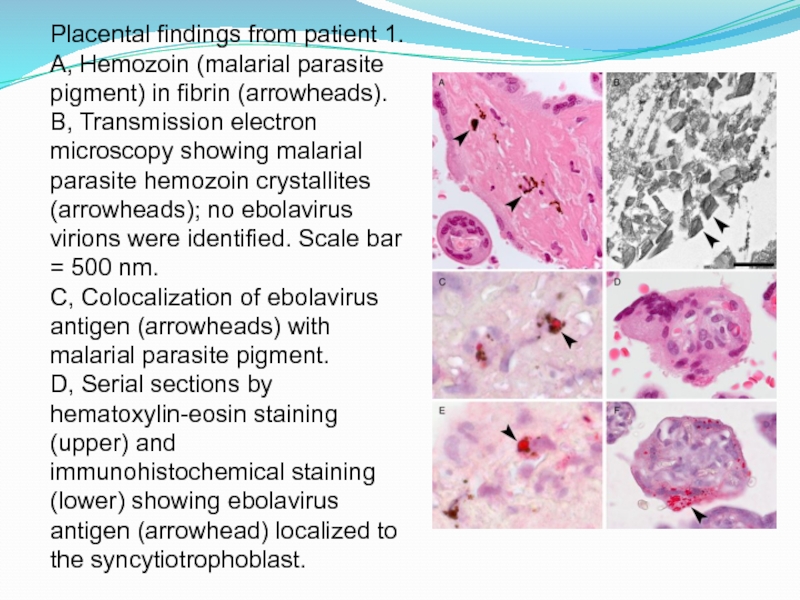
Слайд 15Placental findings from patient 1. A, Hemozoin (malarial parasite pigment)
in fibrin (arrowheads). B, Transmission electron microscopy showing malarial parasite
hemozoin crystallites (arrowheads); no ebolavirus virions were identified. Scale bar = 500 nm.
C, Colocalization of ebolavirus antigen (arrowheads) with malarial parasite pigment.
D, Serial sections by hematoxylin-eosin staining (upper) and immunohistochemical staining (lower) showing ebolavirus antigen (arrowhead) localized to the syncytiotrophoblast.
Слайд 16RESULTS. Patient 2
Patient 2, 29-year-old housewife, who was transferred from
a health center because of suspicion of EVD by a
local clinician who was aware that her relative died recently. She was admitted to the Ebola treatment center on day 4 of illness with fever, fatigue, headache, abdominal pain (with uterine contractions), anorexia, dysphagia, vomiting, diarrhea, and muscle and joint pain. Her last menstrual period date was unknown, but she was initially estimated to be 7 months pregnant. Conjunctival injection was noted. Her heart rate was 80 beats/minute, and her respiratory rate was 20 breaths/minute. Her cervix was 50% effaced with a 4-cm dilation, and fetal movement was normal.
On day 5 of illness, her cervix was 100% effaced with an 8-cm dilation, and she was treated with oxytocin. A malaria rapid diagnostic test was positive, and AL was continued. That night (day 6 of illness), spontaneous vaginal delivery of a live-born male infant occurred without assistance. The degree of vaginal bleeding did not seem abnormal for a normal delivery, although she had had an episode of black stool some hours later. She was treated with oxytocin, ergometrine, intravenous fluids, and cefixime, and Plumpy′nut (Nutriset) was provided. On day 7, the mother's condition rapidly deteriorated, with wheezing, drowsiness, weakness, and a temperature of 38.5°C. Antibiotics were switched to ceftriaxone. On day 8, she became comatose and died. A postmortem skin sample was collected from the mother as part of the routine outbreak response protocol [17].
Слайд 17The infant appeared healthy at birth, with Apgar scores of
8/10/10, and was clinically assessed to be at term on
the basis of examination of the nails and soles of the feet. Infant formula was provided, although the baby may have briefly breastfed immediately after delivery. A placental sample was collected to evaluate for BDBV. Blood collected at 1 day of age (the second day of life) was positive for BDBV by RT-PCR, with a cycle threshold (Ct) of 29.2. Over the next few days, the baby was noted to be quiet and inactive. He became febrile (temperature, 38.5°C) on day 4 of age, and repeat testing of the blood revealed an RT-PCR Ct of 17.9 with negative IgM and IgG ELISA results. Over the next few days, the baby had hematemesis and bloody stools. He developed respiratory distress and coma and died on the seventh day of age (eighth day of life). No postmortem specimens were collected from the infant.
Слайд 18Pathologic Findings
In the placenta, scattered atypical maternal macrophages were seen
within the intervillous space. These cells had degenerate-appearing nuclei, cytoplasmic
blebs, and small eosinophilic cytoplasmic granules, suggestive of viral inclusions (Figure 2A). The placenta was otherwise normal, and the placental membranes and umbilical cord were not sampled. No malarial parasite pigment or parasitized erythrocytes were seen. No virions were seen by transmission electron microscopy.
Ebolavirus antigen was seen by immunohistochemical analysis within the circulating large atypical maternal mononuclear cells (Figure 2B). Antigen was also present in multiple foci within the villous syncytiotrophoblast (Figure 2C), frequently most intense at the basal aspect. Fetal stromal and endothelial cells were negative by immunohistochemical analysis. In the basal plate, immunostaining was prominent within the extravillous trophoblast (Figure 2D) ,with scattered additional cell types likely representing decidual and maternal mononuclear cells. Focally, the lining cells of the maternal vessels of the basal plate (likely endovascular trophoblasts) were positive. Within the placenta, fetal stromal tissue, including villous blood vessels, was negative by immunohistochemical analysis. The postmortem maternal skin specimen was morphologically normal and immunohistochemically negative.
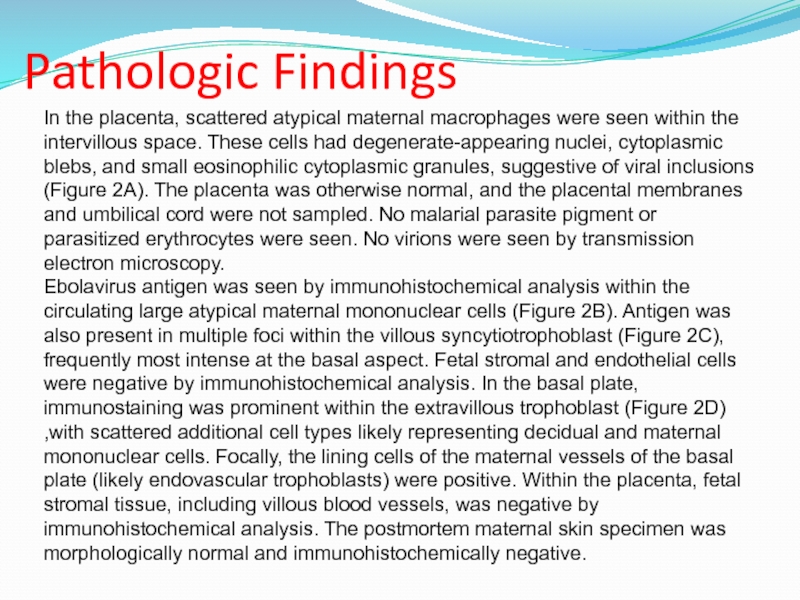
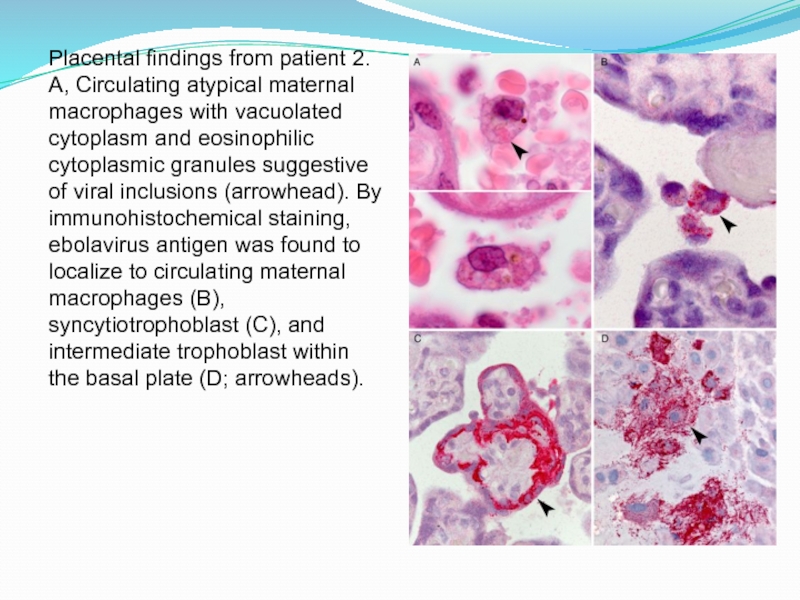
Слайд 19Placental findings from patient 2. A, Circulating atypical maternal macrophages
with vacuolated cytoplasm and eosinophilic cytoplasmic granules suggestive of viral
inclusions (arrowhead). By immunohistochemical staining, ebolavirus antigen was found to localize to circulating maternal macrophages (B), syncytiotrophoblast (C), and intermediate trophoblast within the basal plate (D; arrowheads).
Слайд 20DISCUSSION
Vertical transmission of pathogens can be by transplacental, transvaginal, or
by breastfeeding routes. Placenta sampling provides the opportunity to study
disease processes in living patients and gain insights regarding the mode and mechanism of vertical transmission. In this study, SUDV or BDBV antigen was noted in fetal trophoblast cells, suggesting that these viruses can infect and potentially cross the placental epithelial barrier, resulting in transplacental infection of the fetus. Transplacental infection of the fetus by EBOV has been previously documented in stillbirths by PCR analysis of amniotic fluid, fetal blood, and fetal swab specimens [7, 8]. The immunoprotective role of the placenta may promote the persistence of virus observed in these cases even after virus has been cleared from maternal blood [8, 9].
Several human pathogens can efficiently penetrate the placental barrier and infect the fetus, including some herpesviruses, human immunodeficiency virus, Zika virus, Treponema, and Toxoplasma. The trophoblast is the major cellular barrier to fetal infection, and it comprises 2 major types: the villous trophoblast, which is directly exposed to maternal blood, and the extravillous trophoblast, which invades the maternal decidua and directly contacts maternal cells, including lymphocytes and decidual stromal cells. In this study, both the syncytiotrophoblast (in both patients) and the extravillous trophoblast (in the patient from Isiro) demonstrated ebolavirus antigen by immunohistochemical analysis.
Слайд 24
Types of Body Fluids That involves in transmission of Ebola
virus
BODY FLUIDS
Слайд 26CONTAMINATED OBJECTS THROUGH WHICH EBOLA VIRUS TRANSMITS
Слайд 27IN AFRICA,EBOLA VIRUS MAY BE SPREAD THROUGH BUSHMEAT
Слайд 28
TRADITIONAL AFRICAN RITUALS PLAYED ROLE IN TRANSMISSION OF VIRUS
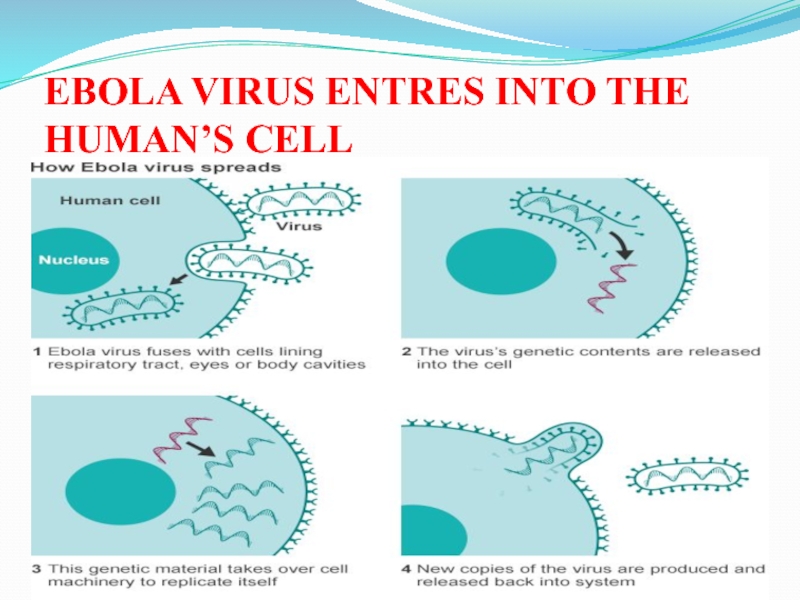
Слайд 29EBOLA VIRUS ENTRES INTO THE HUMAN’S CELL
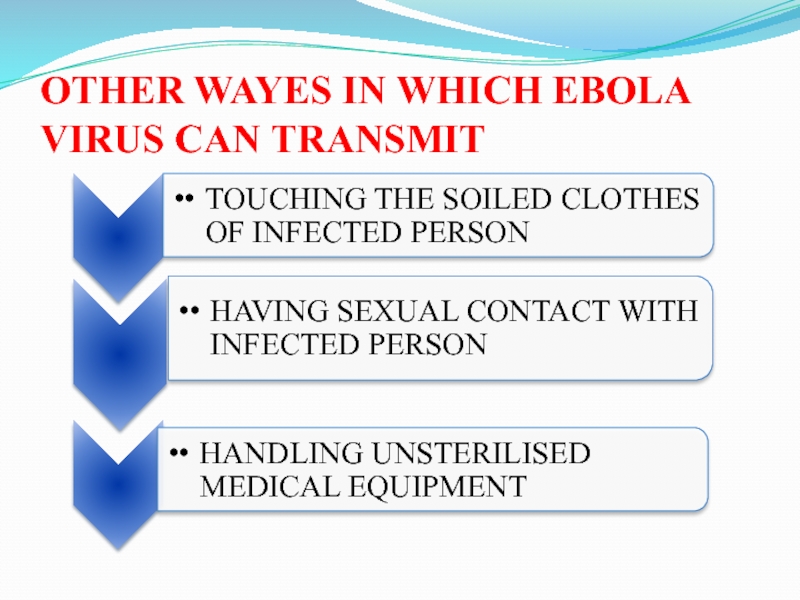
Слайд 30OTHER WAYES IN WHICH EBOLA VIRUS CAN TRANSMIT
Слайд 31
EBOLA VIRUS VICTIM’S BODY BURNED ON HUGE FUNERAL PYRE IN
DESPERATE BATTLE TO STOP OUTBREAK
MASS CREMATION HAVE BEEN
SANCTIONED BY THE GOVERNMENT IN LIBERIA IN BID TO HELP TO HALT THE DEADLY VIRUS
Слайд 32THESE SHOCKING PICTURES SHOW THE BODIES OF EBOLA VICTIMS BEING
BURNED ON HUGE FUNERAL PYRE
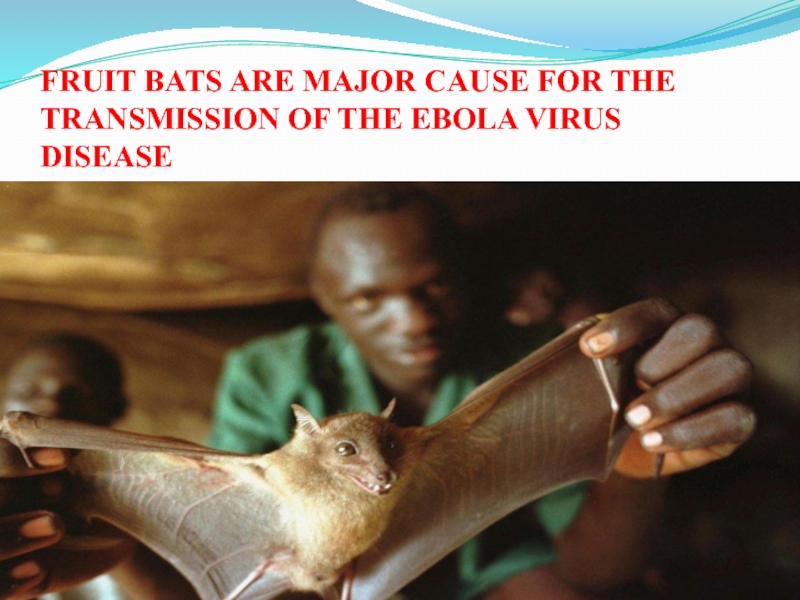
Слайд 33FRUIT BATS ARE MAJOR CAUSE FOR THE TRANSMISSION OF THE
EBOLA VIRUS DISEASE
Слайд 34UNHYGIENIC ENVIRONMENT MAY ALSO BE A CAUSE OF TRANSMISSION OF
EBOLA VIRUS IN WEST AFRICA
Слайд 35CDC WORKER INCINERATES MEDICAL WASTE FROM EBOLA PATIENTS IN ZAIRE
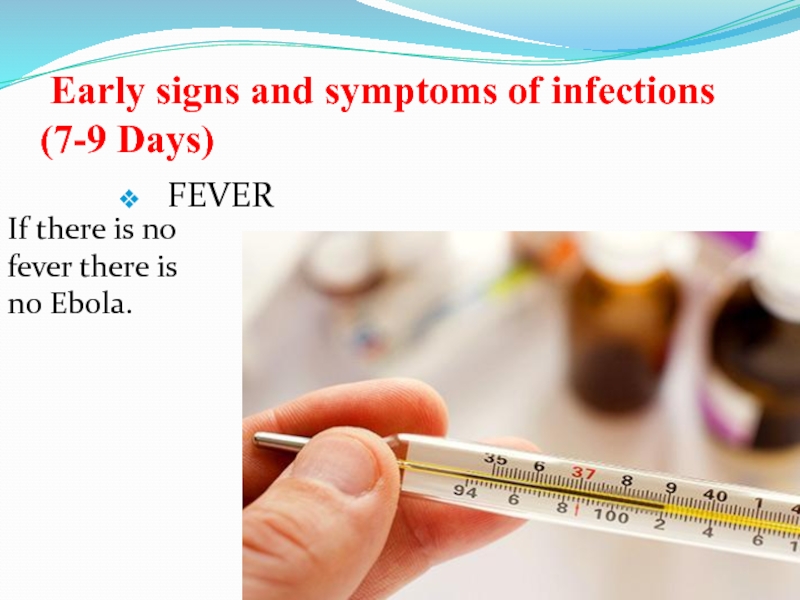
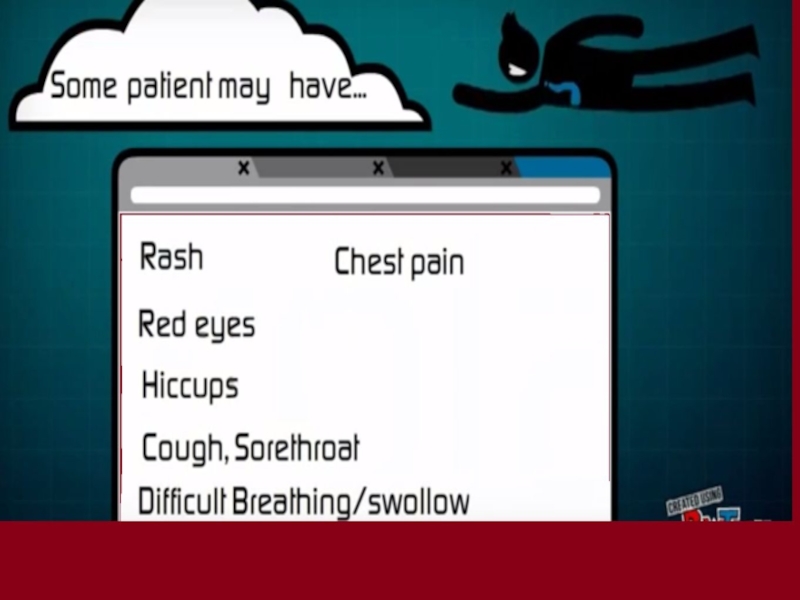
Слайд 38 Early signs and symptoms of infections (7-9 Days)
FEVER
If there
is no fever there is no Ebola.
Слайд 39HEADACHE
Severe headaches start developing
Слайд 40NAUSEA
Sickness in the stomach
and involuntarily impulse
to vomit is
felt by patient.
Слайд 41
MUSCULAR PAIN
Joint and muscle pain leads to intense weakness
throughout the body of the person.
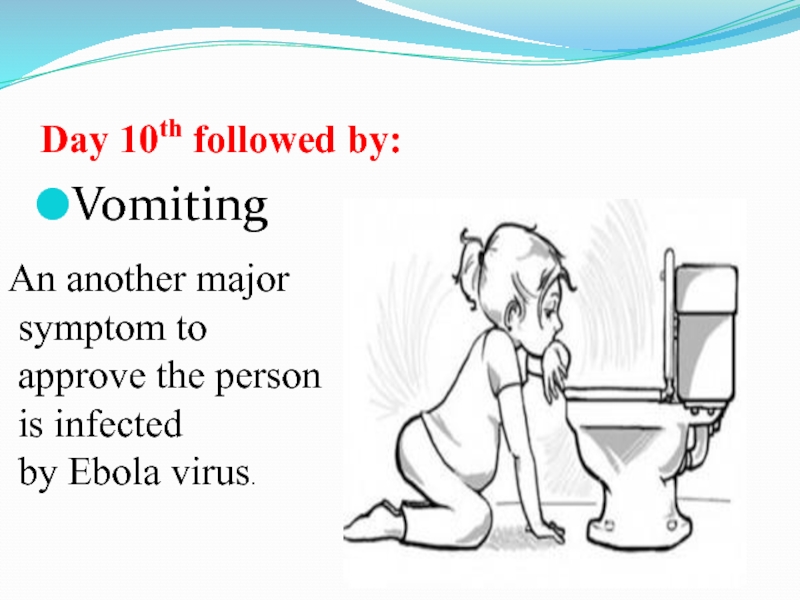
Слайд 44Day 10th followed by:
Vomiting
An another major
symptom to
approve the
person
is infected
by Ebola virus.
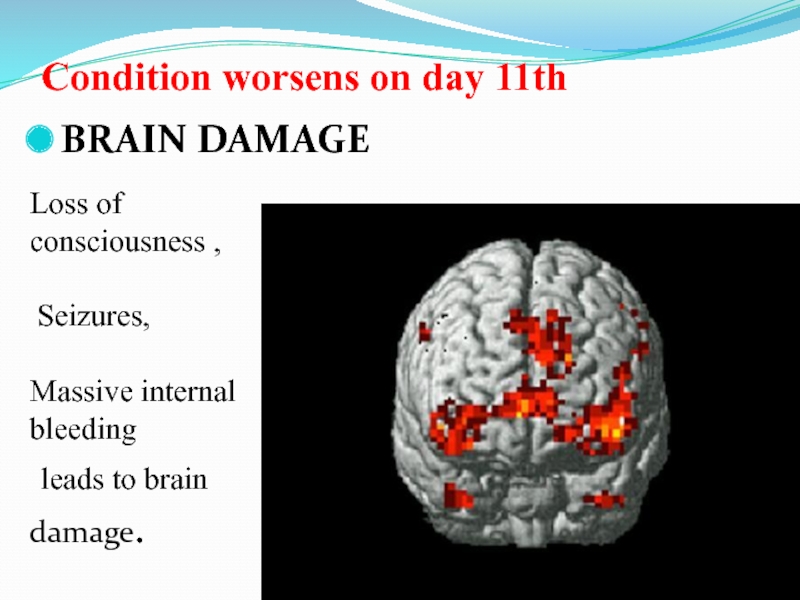
Слайд 47Condition worsens on day 11th
BRAIN DAMAGE
Loss of consciousness ,
Seizures,
Massive
internal bleeding
leads to brain damage.
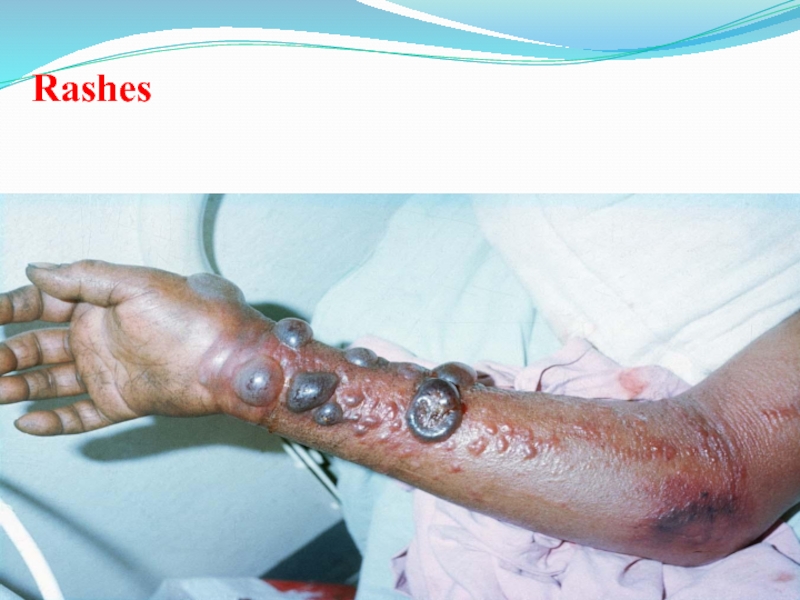
Слайд 48Internal & External Bleeding
Bleeding from body
Openings (nose, gums ,gastrointestinal
tract, etc) may be seen
In some patients.
Слайд 49Diagnosis Of Ebola Virus
How it is diagnosed?
Слайд 50Diagnosis before testing is completed for Ebola, test for following
disease must be completed
Malaria
Typhoid fever
Shigellosis
Cholera
Leptospirosis
Rickettsiosis
Relapsing fever
Meningitis
Hepatitis
Other viral hemorrhagic fevers
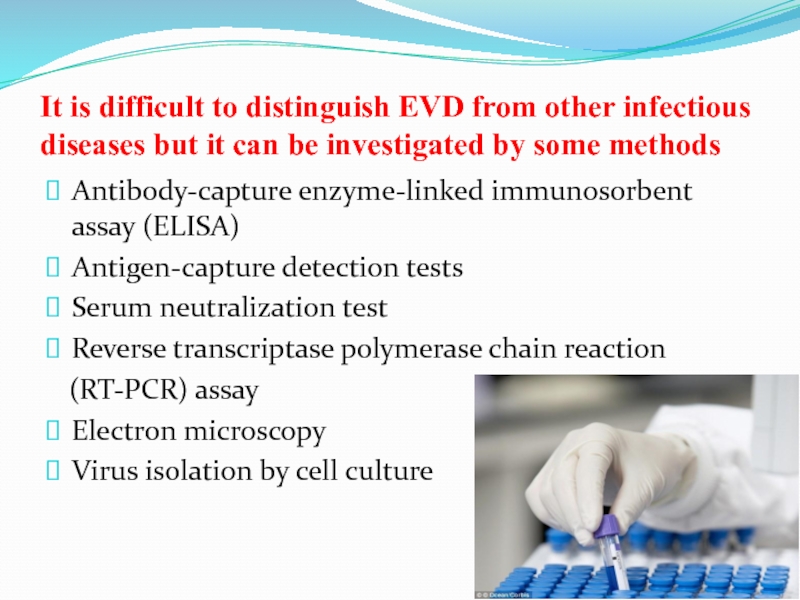
Слайд 51It is difficult to distinguish EVD from other infectious diseases
but it can be investigated by some methods
Antibody-capture enzyme-linked
immunosorbent assay (ELISA)
Antigen-capture detection tests
Serum neutralization test
Reverse transcriptase polymerase chain reaction
(RT-PCR) assay
Electron microscopy
Virus isolation by cell culture
Слайд 52Diagnostic Considerations
Although there is no approved specific therapy for Ebola
virus.
Clinical Findings - include fever of greater than 36.8 C
(101.5 F) and additional signs or symptoms like severe headache, muscle pain, vomiting, diarrhea, abdominal pain
Risk factors
Those who have had contact with blood, body fluids or human remains of a patient known to have or suspected to have Ebola virus disease.
Residence in or travel to an area where Ebola virus transmission is active.
Direct handling of bats, rodents or primates from endemic areas.
Слайд 53A high risk exposure includes any of the following
Percutaneous or
mucous membrane exposure to blood or body fluids of a
person with Ebola virus disease.
Direct skin contact with patient having Ebola virus disease without appropriate personal protective equipment.
Direct contact with dead body without personal safety equipments in a country where an Ebola virus disease outbreak is occurring.
Слайд 54Diagnostic Tests
Rapid blood tests for Marburg and Ebola virus infection
are the most commonly used tests for diagnosis.
Testing for
Ebola and Marburg virus should only be performed in specialized laboratories.
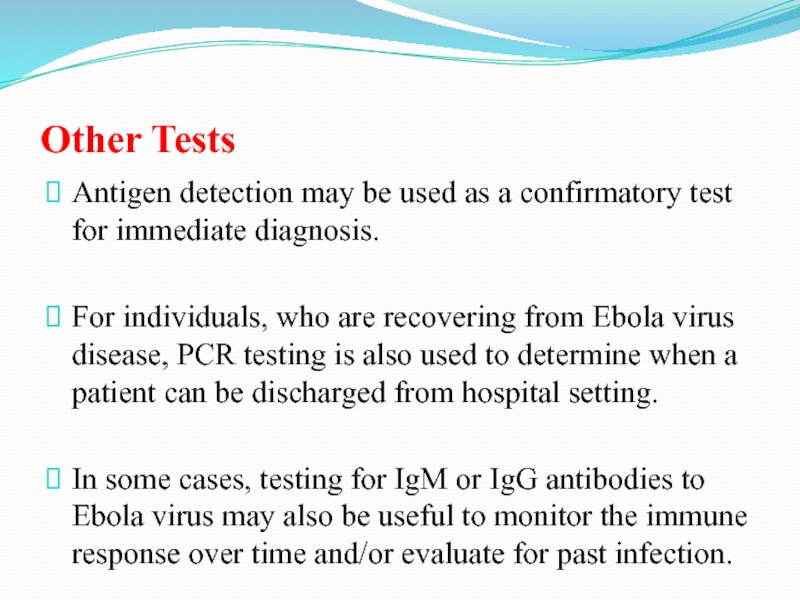
Слайд 55Other Tests
Antigen detection may be used as a confirmatory test
for immediate diagnosis.
For individuals, who are recovering from Ebola virus
disease, PCR testing is also used to determine when a patient can be discharged from hospital setting.
In some cases, testing for IgM or IgG antibodies to Ebola virus may also be useful to monitor the immune response over time and/or evaluate for past infection.
Слайд 56Stages of symptoms of Ebola virus
Stage 1
Headache, sore
throat, fever, muscle soreness
Stage 2
High fever, Vomiting, Passive
Behaviour
Stage 3
Bruising, Bleeding from nose, mouth, eyes;
Blood in stool, Impaired liver function
Stage 4
Loss of consciousness, Seizures, Internal bleeding leading to death
Слайд 57Hospital Protocol for Ebola hit
Handling Personal Protective Equipment (PPE)
Removal
Isolation
Fluid Control
Disinfecting
No
Needles
Слайд 58A new drug target for Ebola virus
Researchers have recently developed
a new drug target in the Ebola virus that could
be used against it to fight the disease
University of Utah chemists have produced a molecule known as peptide mimic that displays a functionally critical region of the virus that is universally conserved in all known species of Ebola
Слайд 59TREATMENT AND VACCINE FOR EBOLA VIRUS
Coffee, Fermented Soy, homeopathic
Spider Venom, And
Vitamin C, May All Hold
Promise As Anti Ebola Virus
Therapies.
Слайд 60Entry point
Ebola infection enters there to be examined by
medical staff in protective gear
Patients are into two groups
based on the probability
Low probability ward
An Ebola Treatment Centre
Слайд 61Patients could face a long wait until their test results
from the lab come back, revealing whether or not they
are infected . Patients who might not have the deadly virus are isolated from those suffering from Ebola , reducing their exposure to the infection while In the treatment centre.
Слайд 62Patients suspected of having Ebola based on the initial medical
examination remain here until official confirmation arrives that they have
the virus . Only once the Ebola diagnosis is confirmed they transferred to another ward
High Probability Ward
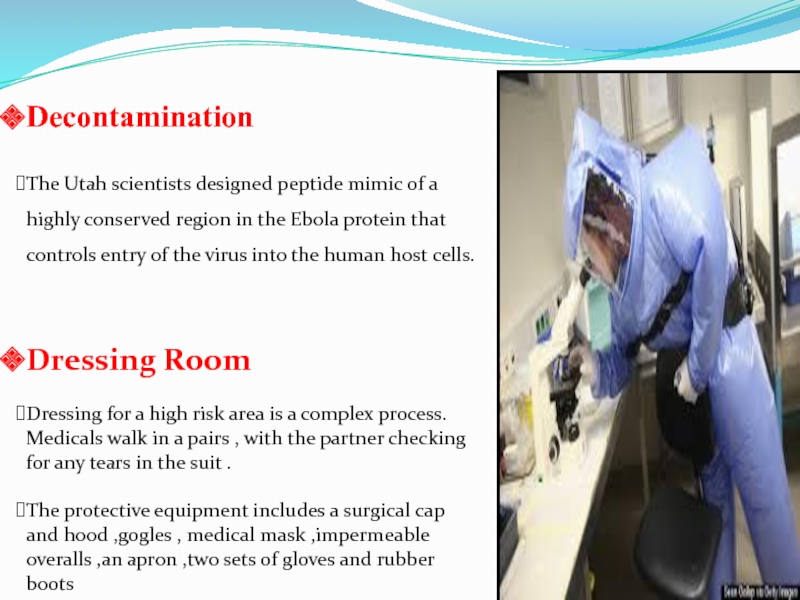
Слайд 63Decontamination
The Utah scientists designed peptide mimic of a highly conserved
region in the Ebola protein that controls entry of the
virus into the human host cells.
Dressing Room
Dressing for a high risk area is a complex process. Medicals walk in a pairs , with the partner checking for any tears in the suit .
The protective equipment includes a surgical cap and hood ,gogles , medical mask ,impermeable overalls ,an apron ,two sets of gloves and rubber boots
Слайд 64Mortuary
The mortuary is located outside the clinic but within the
double fence as bodies are highly infectious .
Patient Exit
The exit on the side are for patients whose blood tests show that they do not have ebola ,or those that recover.
Слайд 65
Statins should be considered as a possible treatment for Ebola
Statins
also have been suggested as a treatment for patient with
sepsis, a condition that involves an out of control immune response similar to that seen in Ebola patients.
Could Statins Treat Ebola
Слайд 66Experimental Canadian made Ebola vaccine is beginning clinical trials in
healthy humans .
The results are expected in December
Clinical trails are
now starting for an experimental made in Canada Ebola vaccine amid growing global concern over the disease that’s left more than 4,000 people dead.
Canadian – Made Ebola Vaccine Starting Clinical Trials In Humans
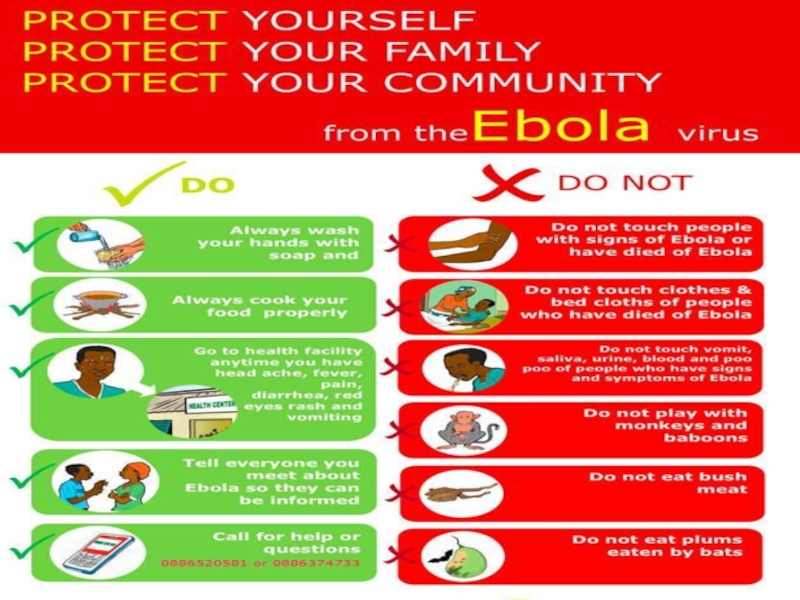
Слайд 68If have sudden fever, diarrhoea, or vomiting, go to the
nearest health facility
Make no contact with Ebola affected people
Use a
special kind of clothes while treating Ebola affected people
HOW TO PREVENT EVD
Слайд 70
QUICK ACCESS TO APPROPRIATE LABORATORY SERVICES
Слайд 71PROPER MANAGEMENT SERVICES FOR WHO ARE INFECTED
Слайд 72PROPER DISPOSAL OF DEAD THROUGH CREMATION OR BURIAL
Слайд 73WORLDWIDE HELPLINE NUMBER
People who go early to the health centre
have a better chance of survival.
CALL 117 WITH YOUR QUESTIONS