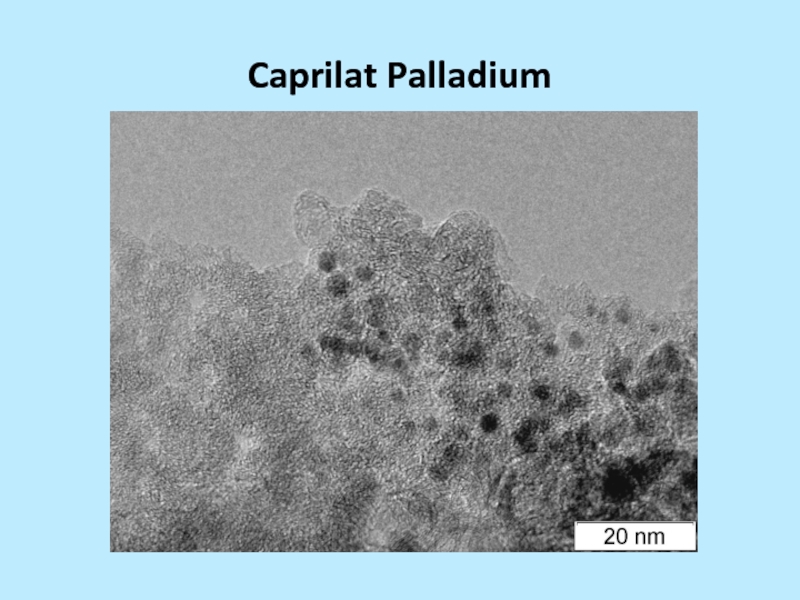
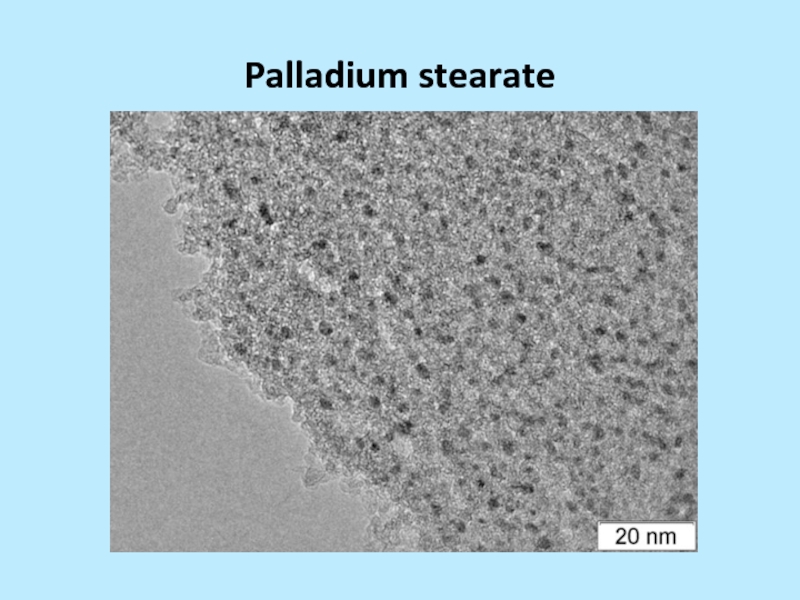
and silver stearate 3-20 nm
Silver Azide 80 nm
Ferrocene and picric
acid 5-20 nmHexogen and iron nitrate 18-55 nm
Ten and cobalt nitrate 15-25 nm
Ten and nickel nitrate 10-20 nm