Слайд 2Combustion, explosions, rusting, rotting, breathing
Слайд 3Oxidation numbers
The oxidation state or oxidation number, is an indicator
of the degree of oxidation (loss of electrons) of an
atom in a chemical compound.
The oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component. This is never exactly true for real bonds.
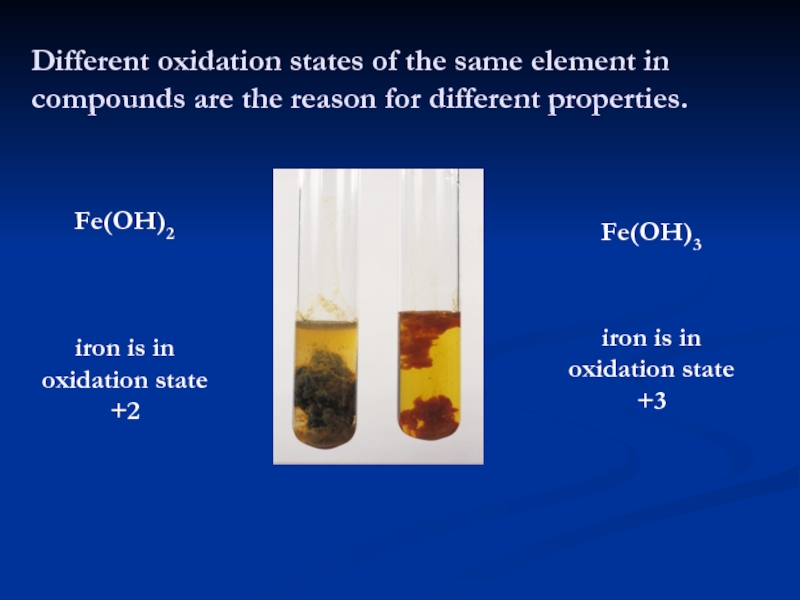
Слайд 4Different oxidation states of the same element in compounds are
the reason for different properties.
Fe(OH)2
Fe(OH)3
iron is in oxidation state
+2
iron is in oxidation state
+3
Слайд 6Determining the oxidation state or number
Any pure element—even if it
forms diatomic molecules like chlorine (Cl2)—has an oxidation state of
zero.
For monatomic ions, the oxidation state is the same as the charge of the ion. E.g., the chloride anion (Cl−) has an oxidation state of −1, whereas the lithium cation (Li+) has an oxidation state of +1.
The sum of oxidation states for all atoms in a polyatomic ion is equal to the charge of the ion. Thus, the oxidation state of one element can be calculated from the oxidation states of the other elements.
The sum of the oxidation states of all atoms in a neutral molecule must be zero.
Слайд 7Rules based on electronegativity
Fluorine in compounds has an oxidation state
of −1.
Halogens other than fluorine have an oxidation state of
−1 except when they are bonded to oxygen, to nitrogen, or to another halogen that is more electronegative.
Hydrogen has an oxidation state of +1 except when bonded to more electropositive elements such as sodium, aluminium, and boron.
In compounds, oxygen typically has an oxidation state of −2.
Alkali metals have an oxidation state of +1 in virtually all of their compounds.
Alkaline earth metals have an oxidation state of +2 in virtually all of their compounds.
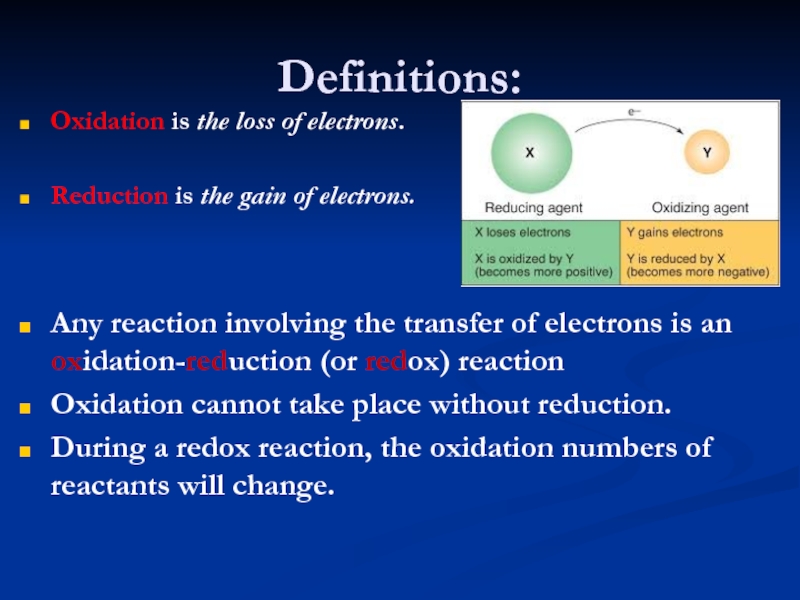
Слайд 8Definitions:
Oxidation is the loss of electrons.
Reduction is the gain of
electrons.
Any reaction involving the transfer of electrons is an oxidation-reduction
(or redox) reaction
Oxidation cannot take place without reduction.
During a redox reaction, the oxidation numbers of reactants will change.
Слайд 9Oxidizing agents
Substances that have the ability to oxidize other substances
(cause them to lose electrons) are known as oxidizing agents,
oxidants, or oxidizers. The oxidizing agent removes electrons from another substance, and is thus itself reduced. And, because it "accepts" electrons, the oxidizing agent is also called an electron acceptor.
Oxygen is the quintessential oxidizer.
Substances with elements in high oxidation states (e.g., concentrated sulfuric acid H2SO4, potassium permanganate KMnO4, potassium dichromate(VI) K2Cr2O7, manganese(IV) oxide MnO2)
or else highly electronegative elements (O2, F2, Cl2, Br2) that can gain extra electrons by oxidizing another substance.
Слайд 10In the Thermit reaction, shown here, which substance is reduced
and which is oxidized?
Aluminium (Al) removes the oxygen atoms from
the iron(III) oxide (Fe2O3). The heat needed to start the reaction is usually provided by a magnesium fuse.
2Al + Fe2O3 Al2O3 + 2Fe
The iron(III) ions are reduced and the aluminium ions are oxidized.
This reaction produces much heat. It is used in incendiary weapons and in underwater welding.
Слайд 11Reducing agents
Substances that have the ability to reduce other substances
(cause them to gain electrons) are said to be reductive
or reducing and are known as reducing agents, reductants, or reducers. They transfer electrons to another substance, and are thus oxidized. And, because it "donates" electrons, the reducing agent is also called an electron donor.
Electropositive elemental metals, such as Li, Na, Mg, Fe, Zn, and Al. These metals donate or give away electrons readily.
C, CO, H2
Слайд 12For any equation to be balanced:
1. The number of atoms
of each type on the left side of the arrow
must equal the number of atoms of each type to the right of the arrow.
The total charges on the left side of the arrow must equal the total charges to the right of the arrow.
The electrons lost (during oxidation) must equal the electrons gained (during reduction).
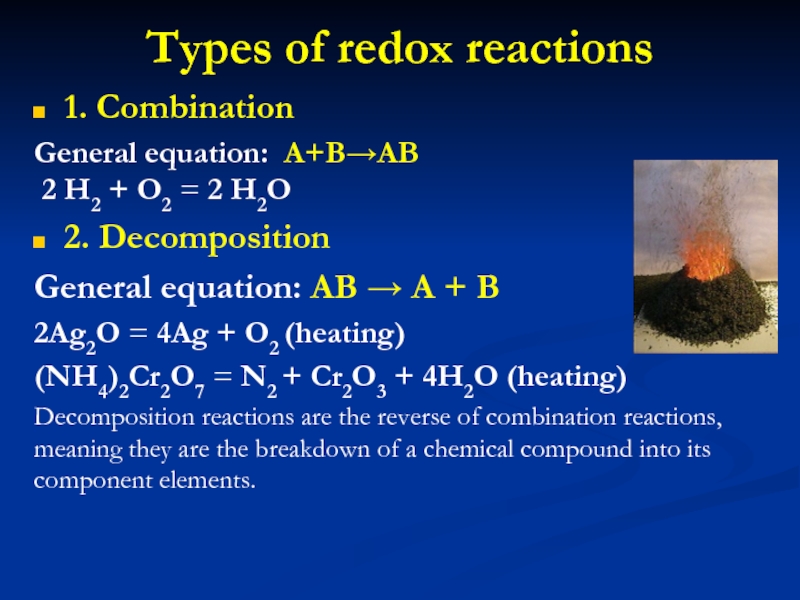
Слайд 13Types of redox reactions
1. Combination
General equation: A+B→AB
2 H2 +
O2 = 2 H2O
2. Decomposition
General equation: AB → A +
B
2Ag2O = 4Ag + O2 (heating)
(NH4)2Cr2O7 = N2 + Cr2O3 + 4H2O (heating)
Decomposition reactions are the reverse of combination reactions, meaning they are the breakdown of a chemical compound into its component elements.
Слайд 14Balancing the equations
K+Mn+7O4-2 + K+Cl- + H2SO4 = Cl20 +
Mn+2SO4 + K2SO4 + H2O
Mn+7 + 5e- = Mn+2
reduction 2
2Cl- - 2e- = Cl20 oxidation 10 5
2 KMnO4 + 10KCl + 8H2SO4 = 5Cl2 + 2MnSO4 + 6K2SO4 + 8H2O
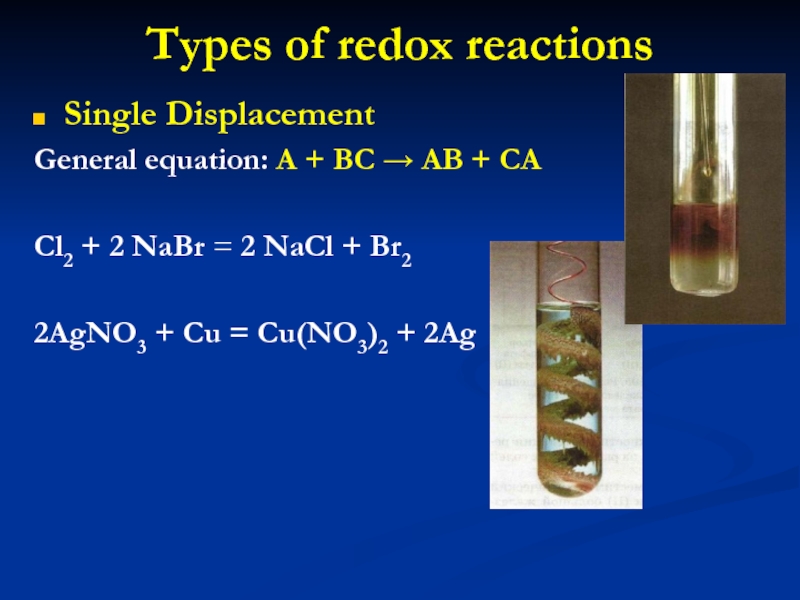
Слайд 15Types of redox reactions
Single Displacement
General equation: A + BC →
AB + CA
Cl2 + 2 NaBr = 2 NaCl +
Br2
2AgNO3 + Cu = Cu(NO3)2 + 2Ag
Слайд 16Types of redox reactions
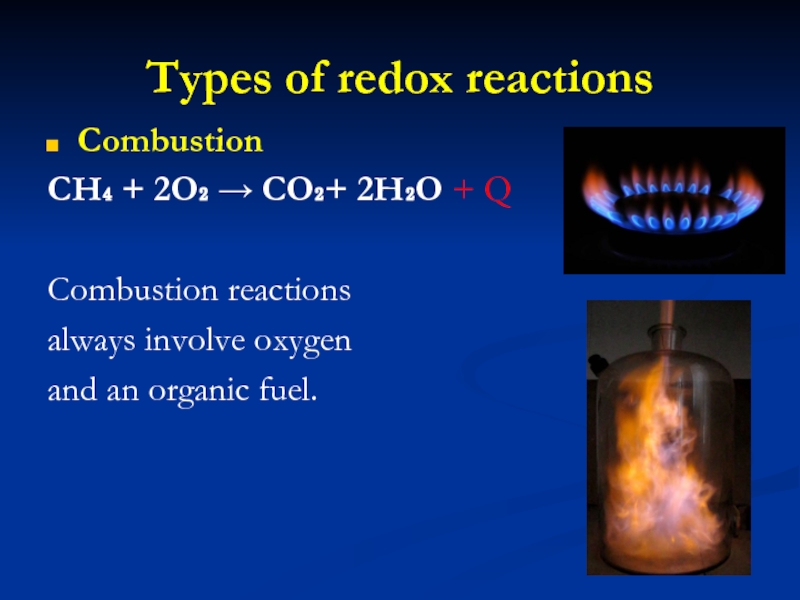
Combustion
CH₄ + 2O₂ → CO₂+ 2H₂O + Q
Combustion
reactions
always involve oxygen
and an organic fuel.
Слайд 17Types of redox reactions
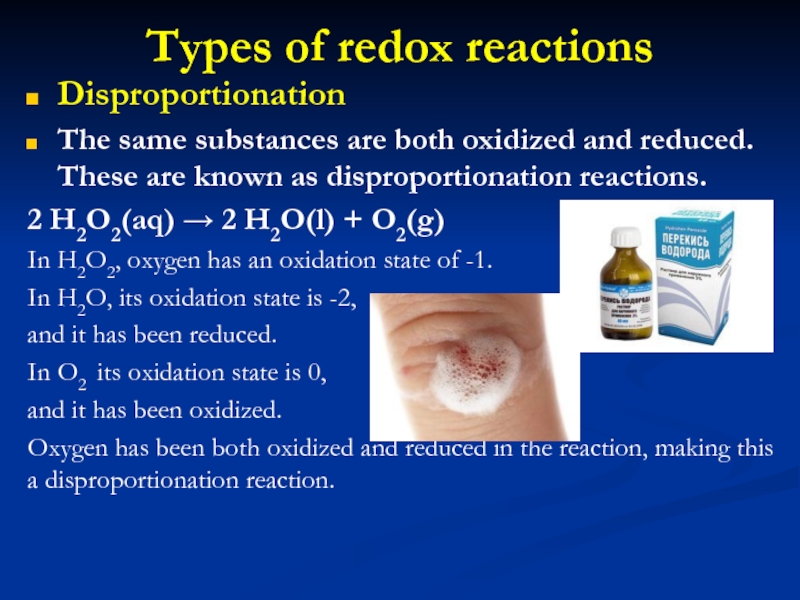
Disproportionation
The same substances are both oxidized and
reduced. These are known as disproportionation reactions.
2 H2O2(aq) →
2 H2O(l) + O2(g)
In H2O2, oxygen has an oxidation state of -1.
In H2O, its oxidation state is -2,
and it has been reduced.
In O2 its oxidation state is 0,
and it has been oxidized.
Oxygen has been both oxidized and reduced in the reaction, making this a disproportionation reaction.
Слайд 18Chemistry IB – Chapter 10, pp.302-335
Balance the equations
NH3 + O2
= NO + H2O
CuO + NH3 = Cu + N2
+ H2O
NO2 + H2O = HNO3 + NO
NH4NO3 = N2O + H2O
K2Cr2O7 + KI + H2SO4 = Cr2(SO4)3 + K2SO4 + I2 + H2O
KMnO4 + HCl = Cl2↑ + MnCl2 + KCl + H2O