Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Строение атомов (Структура атома)
Содержание
- 1. Строение атомов (Структура атома)
- 2. Основные определения атома (the concept of the
- 3. Что такое атом? (What is an atom?)Атом
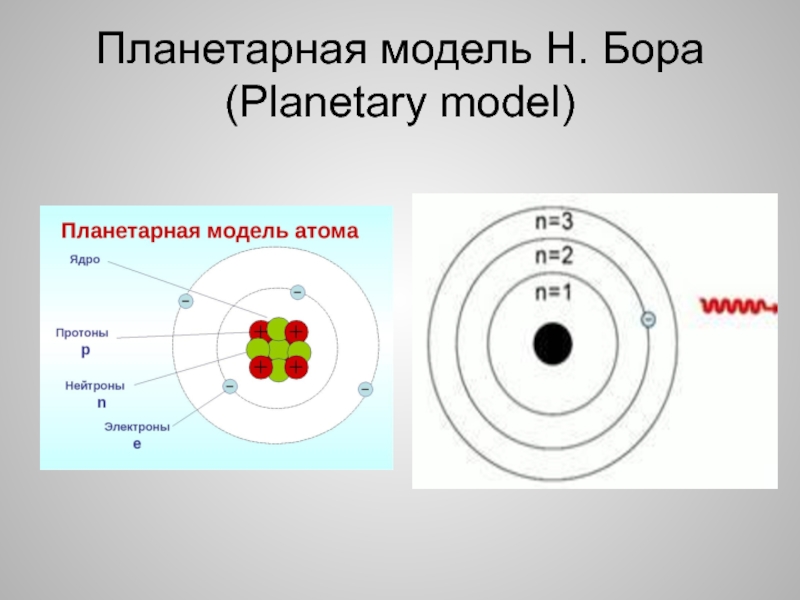
- 4. Планетарная модель Н. Бора (Planetary model)
- 5. Химические элементы – это разные виды атомовВ
- 6. Изотопы (Isotopes)Один вид элементов может содержать разное
- 7. Ядро атомаЯдро атома состоит из протонов и
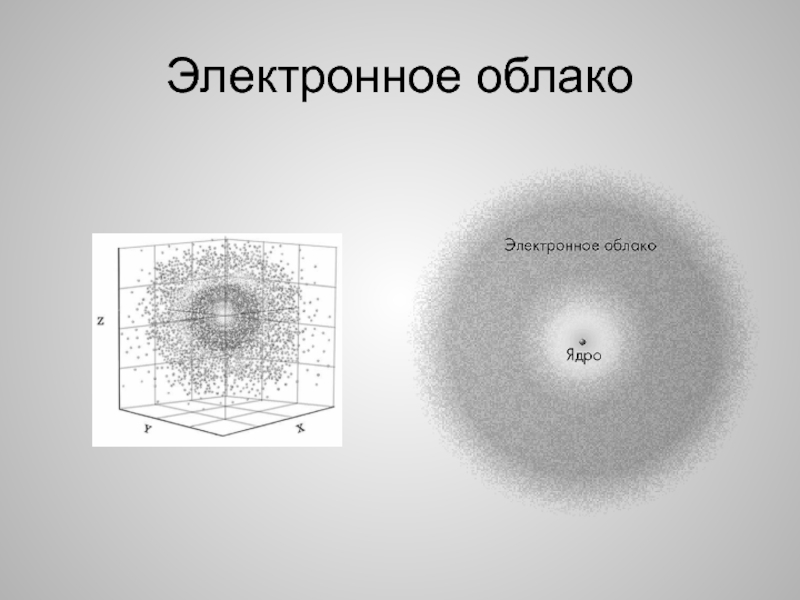
- 8. Электронное облако
- 9. Электронное облако молекулы водорода (Electron cloud of the hydrogen molecule)
- 10. Ядро и его масса (The nucleus and
- 11. Массовое число элемента (mass number)Масса протона
- 12. Основные термины, понятия и определения (Basic terms, concepts and definitions)АтомИзотопПротонНейтронЭлектронНуклонСубатомная частицаМассовое число элементаЭлектронное облакоПланетарная модель атома
- 13. Скачать презентанцию
Слайды и текст этой презентации
Слайд 1Строение атомов
(Структура атома)
The structure of atoms
(atomic structure)
Занятие № 4. Иностранные
студенты. Химико-биологический (медицинский) профиль подготовки
Слайд 2Основные определения атома
(the concept of the atom)
Атом – наименьшая часть
элемента, являющаяся носителем его свойств
Атом – наибольшая микрочастица вещества, сохраняющаяся
при химических превращенияхСвойства атомов не изменяются при химических превращениях
Atom – the smallest part of an element which is the bearer of its properties
Atom – the largest microparticle of a substance remaining during chemical transformations
Properties of atoms are not changed in chemical reactions
Слайд 3Что такое атом?
(What is an atom?)
Атом - сложная частица. Он
состоит из протонов, нейтронов и электронов. Протоны и нейтроны образуют
ядро атома.Электроны могут существовать на строго определенных расстояниях от ядра атома – на стационарных орбитах
Рассмотрите рисунок на следующем слайде
The atom is a complex particle. It consists of protons, neutrons, and electrons. Protons and neutrons form the nucleus of the atom.
Electrons can exist at specific distances from the nucleus of an atom in fixed orbits
Look at the picture on the next slide
Слайд 5Химические элементы – это разные виды атомов
В природе существует 88
видов атомов. Это элементы, которые были найдены на планете Земля
20
видов атомов создано в химических лабораторияхВ Периодической Таблице может быть указано118 или 126 элементов
Chemical elements is different types of atoms
In nature, there are 88 species of atoms. These are elements that were found on the planet Earth
20 types of atoms are created in chemical laboratories
In the Periodic Table can be specified 118 or 126 elements
Слайд 6Изотопы
(Isotopes)
Один вид элементов может содержать разное количество нейтронов и поэтому
различаться своей массой.
Один вид электронов, но с разной массой
называют изотопамиВ Периодической Таблице указывают среднее значение массы всех возможных изотопов элементов
One type elements can contain different numbers of neutrons and therefore differ in their mass.
One kind of electrons but different mass are called isotopes
In the Periodic Table indicate the average mass of all possible isotopes of the elements
http:www.ciaaw.org/pubs/Periodic_Table_Isotopes.pdf.
Слайд 7Ядро атома
Ядро атома состоит из протонов и нейтронов. Эти частицы
называют нуклонами
Электрон занимает вокруг ядра область подобную шару – электронное
облакоНуклоны и электроны называют субатомными частицами
The nucleus of an atom consists of protons and neutrons. These particles are called nucleons
The electron takes around the nucleus, the region is like the ball – the electron cloud
Nucleons and electrons are called subatomic particles
Слайд 10Ядро и его масса
(The nucleus and its mass)
Протоны несут положительный
заряд (+1)
Нейтроны не заряжены
Количество протонов и нейтронов не совпадает
Количество
протонов и нейтронов совпадаетЗаряд ядра (Z) и число электронов совпадает с номером элемента в Периодической Таблице
Protons carry a positive charge (+1)
Neutrons are not charged
The number of protons and neutrons is not the same
The number of protons and neutrons coincide
The nuclear charge (Z) and the number of electrons matches the number of elements in the Periodic Table
Слайд 11Массовое число элемента
(mass number)
Масса протона и нейтрона приблежается к
1 (единице)
Масса электрона по сравнению с нуклонами очень мала (0,000549
а.е.м).Масса атома определяется массой его нуклонов (массовым числом): A=Z+N
The mass of the proton and neutron approaching to 1 (one)
The electron mass compared to the nucleons is very small (0,000549 a.e.m).
The mass of the atom is determined by the mass of its nucleons (mass number): A=Z+N