Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Atomic physics
Содержание
- 1. Atomic physics
- 2. Physical Interpretation of the Quantum NumbersThe Orbital
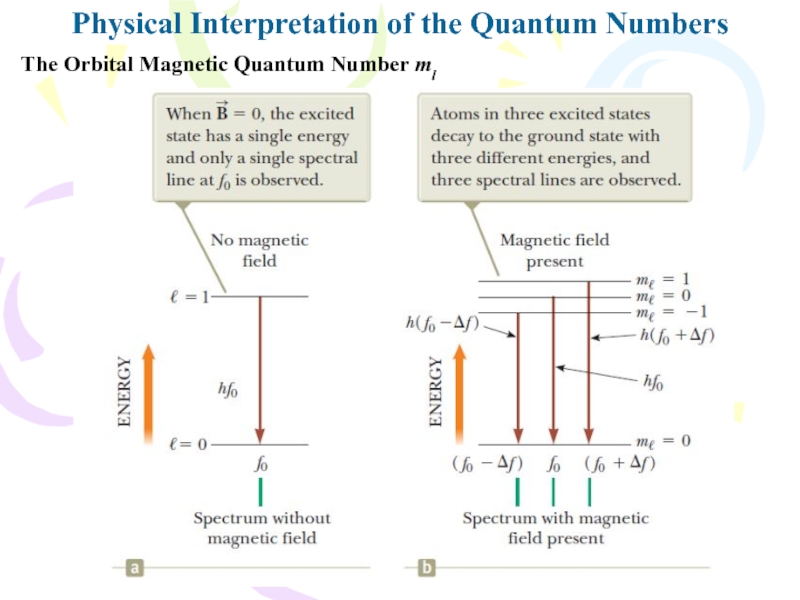
- 3. Physical Interpretation of the Quantum NumbersThe Orbital Magnetic Quantum Number ml
- 4. Physical Interpretation of the Quantum NumbersThe Orbital
- 5. Physical Interpretation of the Quantum NumbersThe Orbital Magnetic Quantum Number ml
- 6. Physical Interpretation of the Quantum NumbersThe Spin Magnetic Quantum Number msElectron spin
- 7. Because spin is a form of angular
- 8. Physical Interpretation of the Quantum NumbersThe Spin Magnetic Quantum Number ms
- 9. Physical Interpretation of the Quantum NumbersThe Spin Magnetic Quantum Number ms
- 10. The Exclusion Principle and the Periodic TableFor
- 11. The Exclusion Principle and the Periodic TableThe
- 12. The Exclusion Principle and the Periodic TableHund’s
- 13. The Exclusion Principle and the Periodic TableThe periodic table
- 14. The Exclusion Principle and the Periodic TableIonization energy of the elements versus atomic number
- 15. More on Atomic Spectra: Visible and X-RayTransitions
- 16. More on Atomic Spectra: Visible and X-RayThe
- 17. More on Atomic Spectra: Visible and X-RayX-Ray
- 18. More on Atomic Spectra: Visible and X-RayX-Ray
- 19. X-Ray SpectraMore on Atomic Spectra: Visible and
- 20. More on Atomic Spectra: Visible and X-RayX-Ray
- 21. More on Atomic Spectra: Visible and X-RayX-Ray
- 22. Spontaneous and Stimulated TransitionsThis process is called
- 23. Spontaneous and Stimulated TransitionsOnce an atom is
- 24. Spontaneous and Stimulated TransitionsIn addition to spontaneous
- 25. LasersThe primary properties of laser light that
- 26. LasersWe have described how an incident photon
- 27. LasersFor the stimulated emission to result in
- 28. Lasers• The emitted photons must be confined
- 29. LasersOne device that exhibits stimulated emission of
- 30. LasersApplicationsThis robot carrying laser scissors, which can
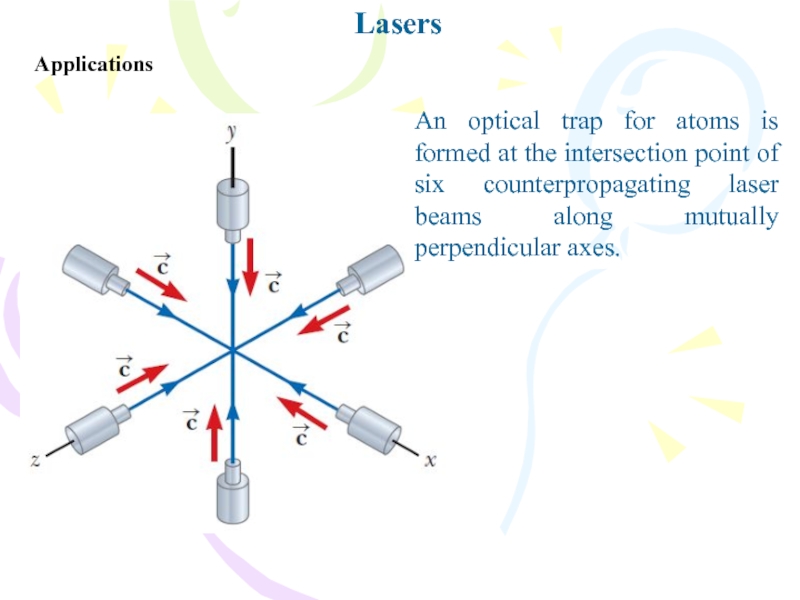
- 31. LasersApplicationsAn optical trap for atoms is formed
- 32. LasersApplicationsAn extension of laser trapping, laser cooling,
- 33. Скачать презентанцию
Слайды и текст этой презентации
Слайд 1Course of lectures «Contemporary Physics: Part2»
Lecture №11
Atomic Physics. Physical Interpretation
Слайд 2Physical Interpretation of the Quantum Numbers
The Orbital Quantum Number l
According
to quantum mechanics, an atom in a state whose principal
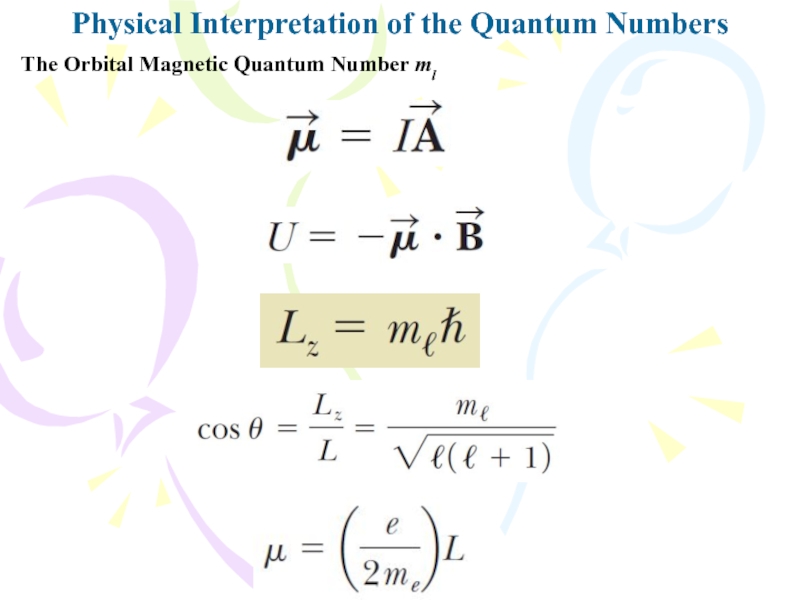
quantum number is n can take on the following discrete values of the magnitude of the orbital angular momentum:Слайд 4Physical Interpretation of the Quantum Numbers
The Orbital Magnetic Quantum Number
ml
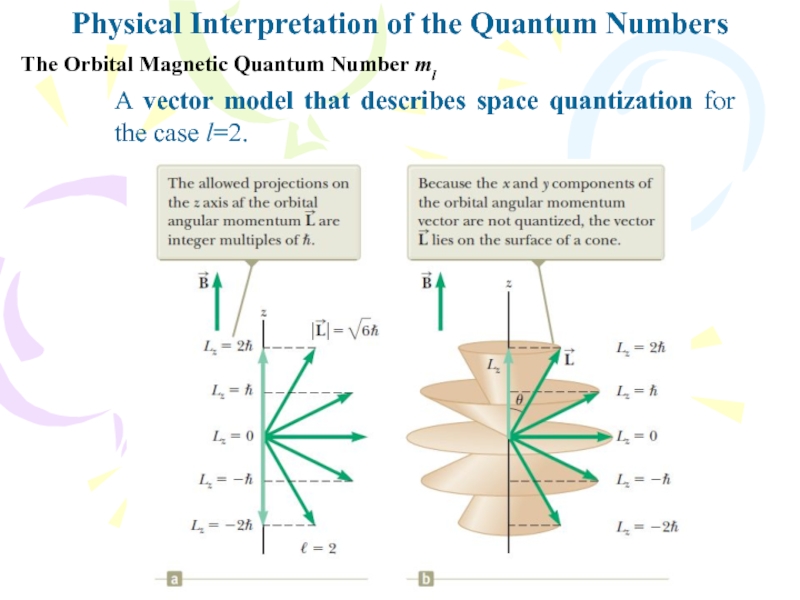
A vector model that describes space quantization for the case
l=2.Слайд 6Physical Interpretation of the Quantum Numbers
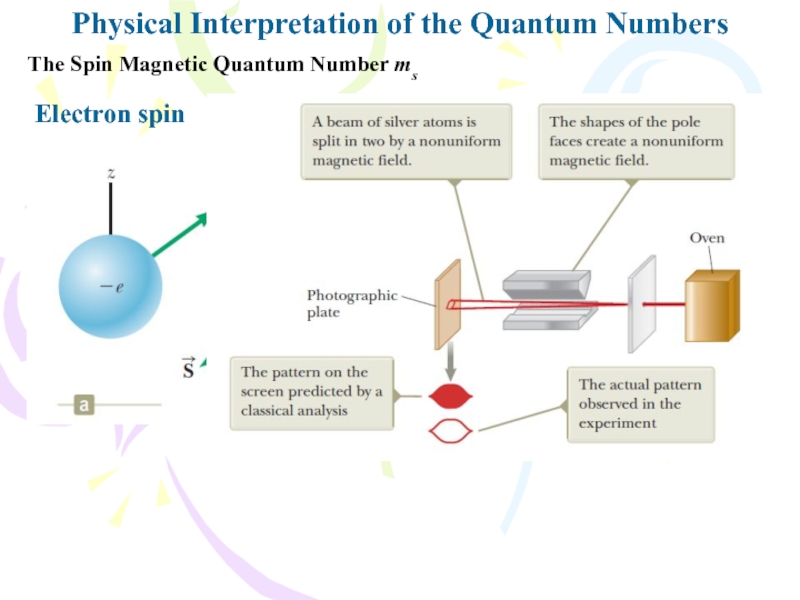
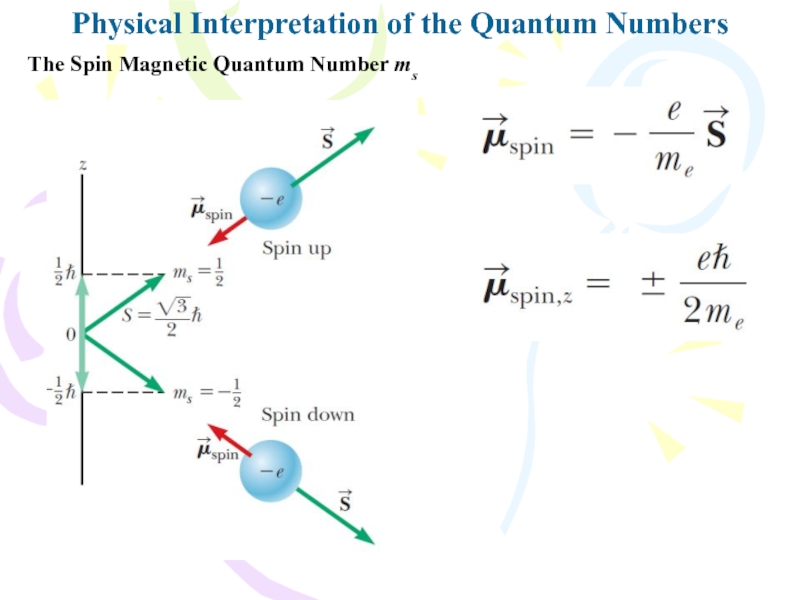
The Spin Magnetic Quantum Number
ms
Electron spin
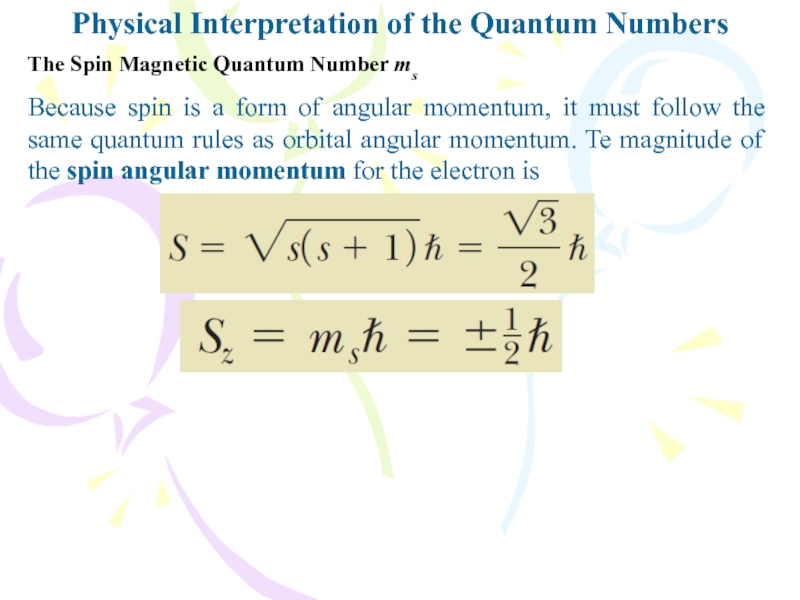
Слайд 7Because spin is a form of angular momentum, it must
follow the same quantum rules as orbital angular momentum. Te
magnitude of the spin angular momentum for the electron isPhysical Interpretation of the Quantum Numbers
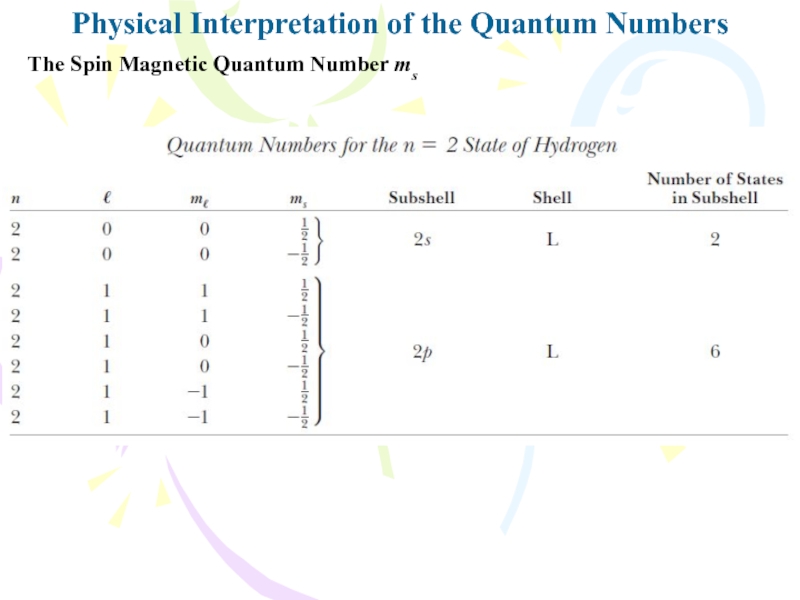
The Spin Magnetic Quantum Number ms
Слайд 10The Exclusion Principle and the Periodic Table
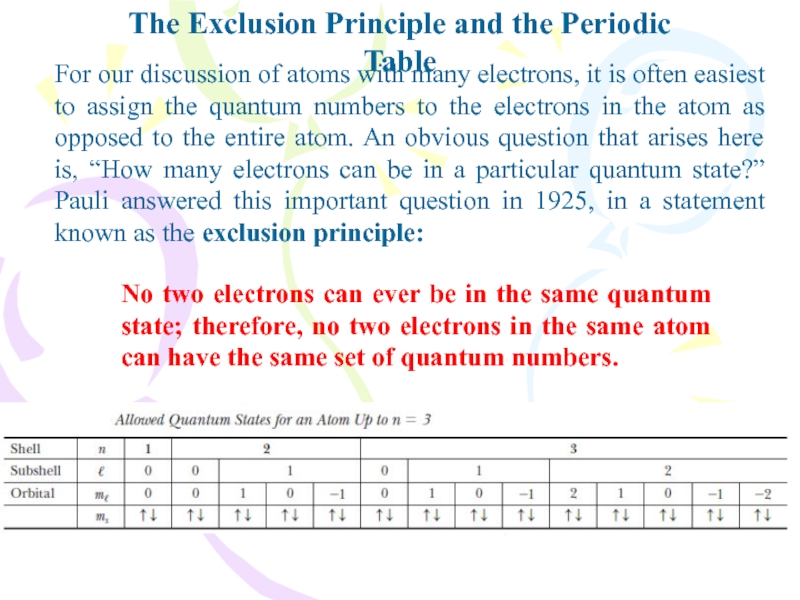
For our discussion of
atoms with many electrons, it is often easiest to assign
the quantum numbers to the electrons in the atom as opposed to the entire atom. An obvious question that arises here is, “How many electrons can be in a particular quantum state?” Pauli answered this important question in 1925, in a statement known as the exclusion principle:No two electrons can ever be in the same quantum state; therefore, no two electrons in the same atom can have the same set of quantum numbers.
Слайд 11The Exclusion Principle and the Periodic Table
The filling of electronic
states must obey both the exclusion principle and Hund’s rule
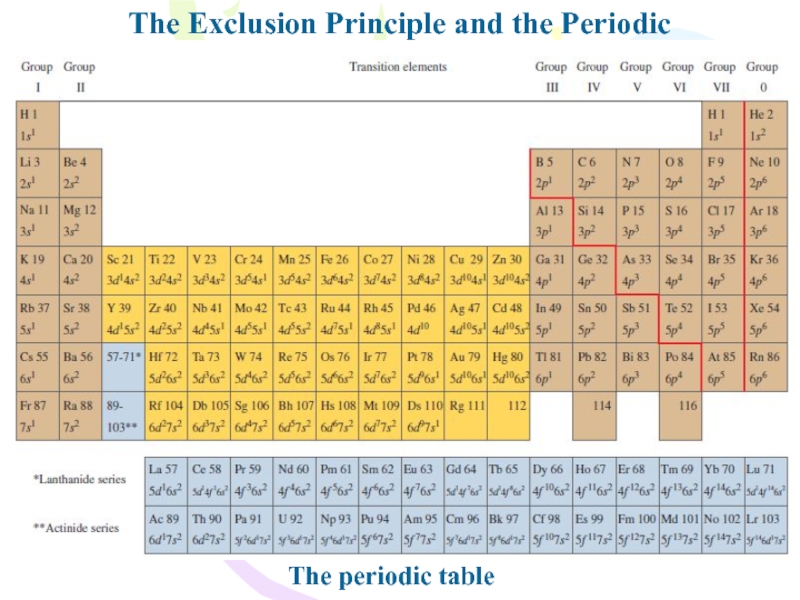
Слайд 12The Exclusion Principle and the Periodic Table
Hund’s rule, states that
when an atom has orbitals of equal energy, the order
in which they are filled by electrons is such that a maximum number of electrons have unpaired spins.Слайд 14The Exclusion Principle and the Periodic Table
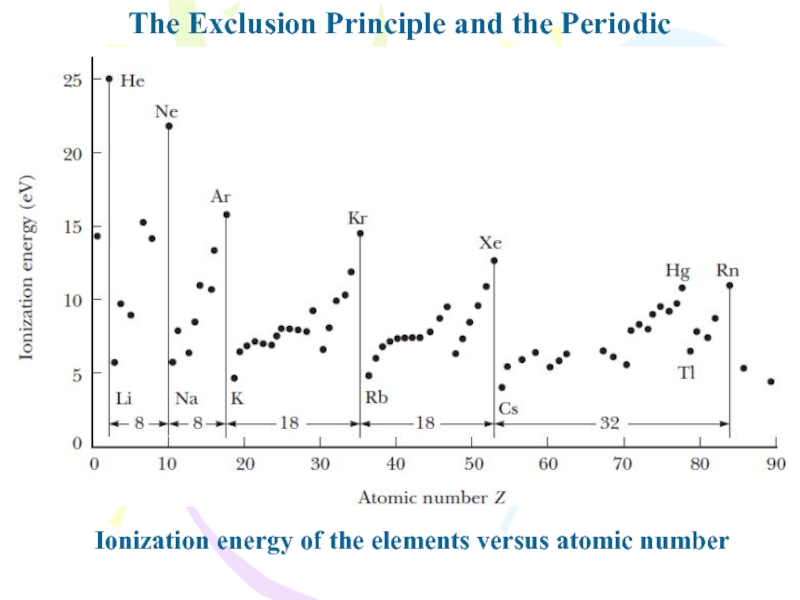
Ionization energy of the
elements versus atomic number
Слайд 15More on Atomic Spectra: Visible and X-Ray
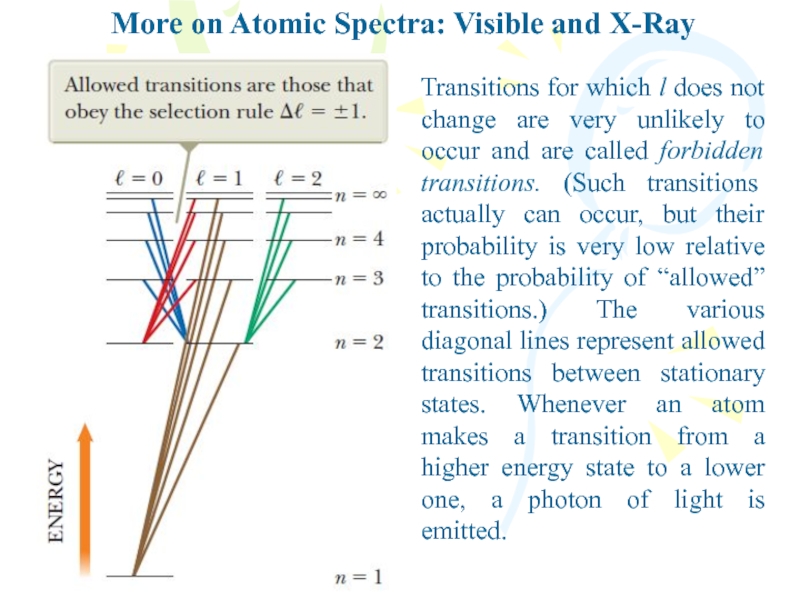
Transitions for which l
does not change are very unlikely to occur and are
called forbidden transitions. (Such transitions actually can occur, but their probability is very low relative to the probability of “allowed” transitions.) The various diagonal lines represent allowed transitions between stationary states. Whenever an atom makes a transition from a higher energy state to a lower one, a photon of light is emitted.Слайд 16More on Atomic Spectra: Visible and X-Ray
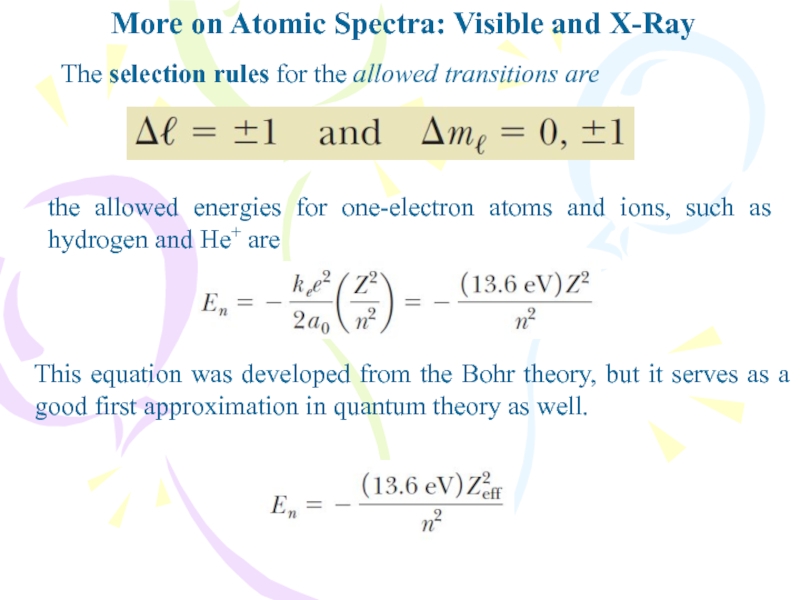
The selection rules for
the allowed transitions are
the allowed energies for one-electron atoms and
ions, such as hydrogen and He+ areThis equation was developed from the Bohr theory, but it serves as a good first approximation in quantum theory as well.
Слайд 17More on Atomic Spectra: Visible and X-Ray
X-Ray Spectra
X-rays are emitted
when high-energy electrons or any other charged particles bombard a
metal target. The x-ray spectrum typically consists of a broad continuous band containing a series of sharp lines.X-ray radiation with its origin in the slowing down of electrons is called bremsstrahlung, the German word for “braking radiation.”
The discrete lines called characteristic x-rays.
Слайд 18More on Atomic Spectra: Visible and X-Ray
X-Ray Spectra
Figure shows a
machine that uses a linear accelerator to accelerate electrons up
to 18 MeV and smash them into a tungsten target. The result is a beam of photons, up to a maximum energy of 18 MeV, which is actually in the gamma-ray range. This radiation is directed at the tumor in the patient.Слайд 19X-Ray Spectra
More on Atomic Spectra: Visible and X-Ray
Other characteristic x-ray
lines are formed when electrons drop from upper levels to
vacancies other than those in the K shell. For example, L lines are produced when vacancies in the L shell are filled by electrons dropping from higher shells. An Lα line is produced as an electron drops from the M shell to the L shell, and an Lβ line is produced by a transition from the N shell to the L shell.Слайд 20More on Atomic Spectra: Visible and X-Ray
X-Ray Spectra
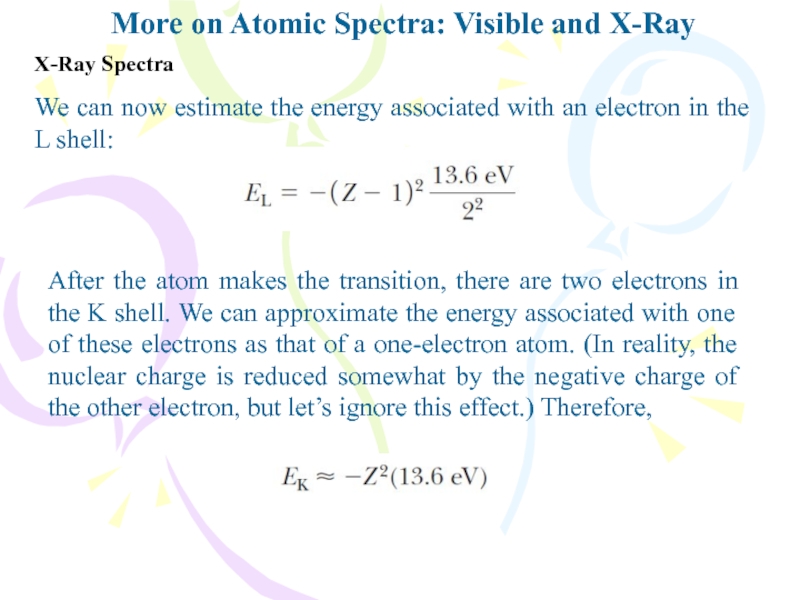
We can now
estimate the energy associated with an electron in the L
shell:After the atom makes the transition, there are two electrons in the K shell. We can approximate the energy associated with one of these electrons as that of a one-electron atom. (In reality, the nuclear charge is reduced somewhat by the negative charge of the other electron, but let’s ignore this effect.) Therefore,
Слайд 21More on Atomic Spectra: Visible and X-Ray
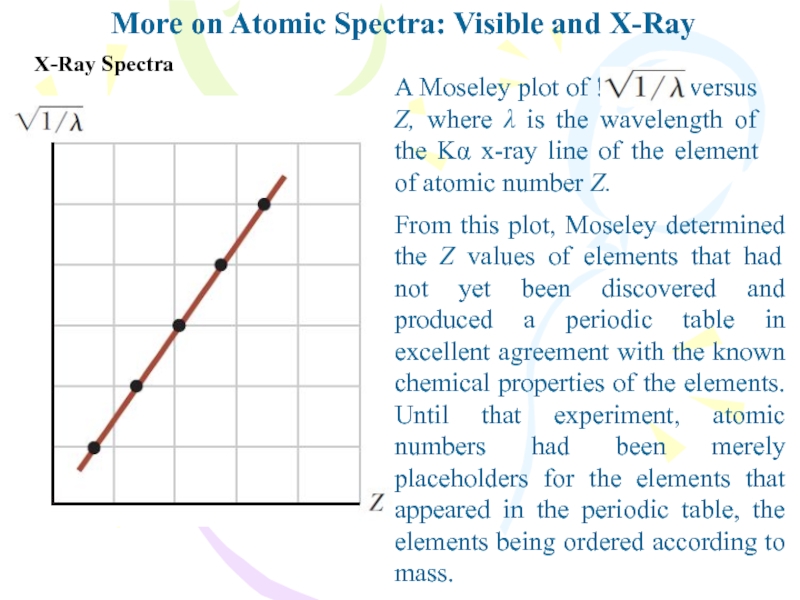
X-Ray Spectra
A Moseley plot
of !1/l versus Z, where λ is
the wavelength of the Kα x-ray line of the element of atomic number Z.From this plot, Moseley determined the Z values of elements that had not yet been discovered and produced a periodic table in excellent agreement with the known chemical properties of the elements. Until that experiment, atomic numbers had been merely placeholders for the elements that appeared in the periodic table, the elements being ordered according to mass.
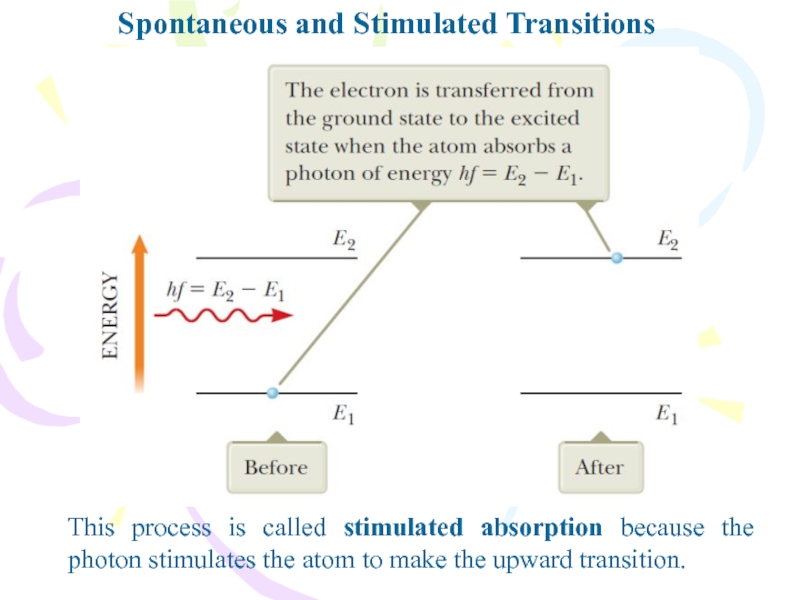
Слайд 22Spontaneous and Stimulated Transitions
This process is called stimulated absorption because
the photon stimulates the atom to make the upward transition.
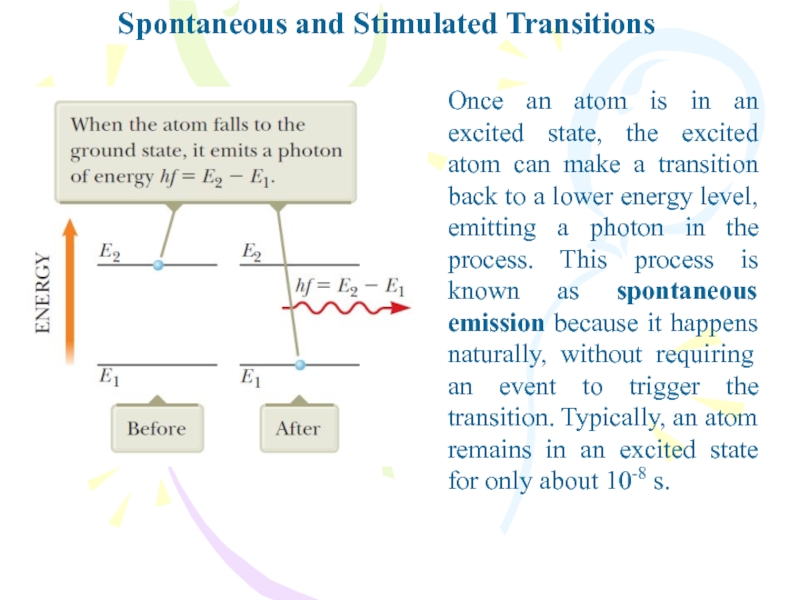
Слайд 23Spontaneous and Stimulated Transitions
Once an atom is in an excited
state, the excited atom can make a transition back to
a lower energy level, emitting a photon in the process. This process is known as spontaneous emission because it happens naturally, without requiring an event to trigger the transition. Typically, an atom remains in an excited state for only about 10-8 s.Слайд 24Spontaneous and Stimulated Transitions
In addition to spontaneous emission, stimulated emission
occurs. In this process, the incident photon is not absorbed;
therefore, after the stimulated emission, two photons with identical energy exist: the incident photon and the emitted photon.Слайд 25Lasers
The primary properties of laser light that make it useful
in these technological applications are the following:
• Laser light is
coherent. The individual rays of light in a laser beam maintain a fixed phase relationship with one another.• Laser light is monochromatic. Light in a laser beam has a very narrow range of wavelengths.
• Laser light has a small angle of divergence. The beam spreads out very little, even over large distances.
Слайд 26Lasers
We have described how an incident photon can cause atomic
energy transitions either upward (stimulated absorption) or downward (stimulated emission).
The two processes are equally probable. When light is incident on a collection of atoms, a net absorption of energy usually occurs because when the system is in thermal equilibrium, many more atoms are in the ground state than in excited states. If the situation can be inverted so that more atoms are in an excited state than in the ground state, however, a net emission of photons can result. Such a condition is called population inversion. Population inversion is, in fact, the fundamental principle involved in the operation of a laser (an acronym for light amplification by stimulated emission of radiation). The full name indicates one of the requirements for laser light: to achieve laser action, the process of stimulated emission must occur.Слайд 27Lasers
For the stimulated emission to result in laser light, there
must be a buildup of photons in the system. The
following three conditions must be satisfied to achieve this buildup:• The system must be in a state of population inversion: there must be more atoms in an excited state than in the ground state. That must be true because the number of photons emitted must be greater than the number absorbed.
• The excited state of the system must be a metastable state, meaning that its lifetime must be long compared with the usually short lifetimes of excited states, which are typically 1028 s. In this case, the population inversion can be established and stimulated emission is likely to occur before spontaneous emission.
Слайд 28Lasers
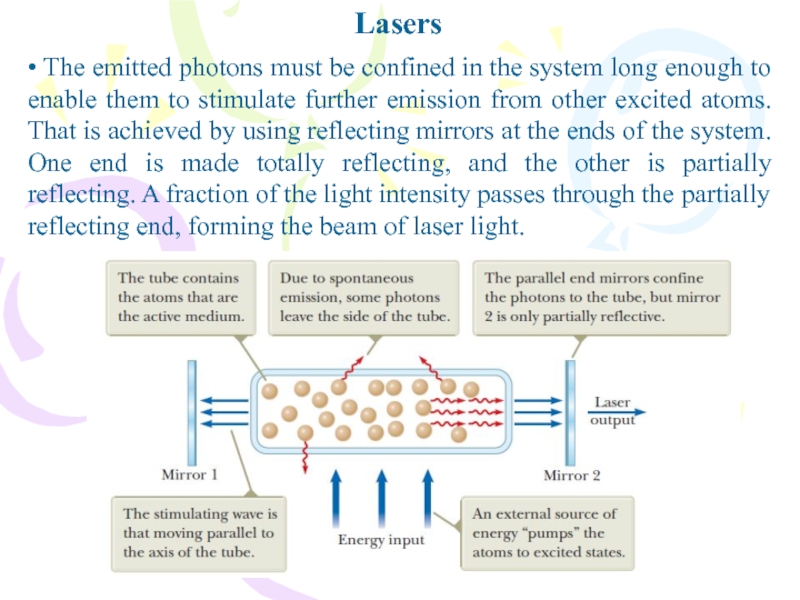
• The emitted photons must be confined in the system
long enough to enable them to stimulate further emission from
other excited atoms. That is achieved by using reflecting mirrors at the ends of the system. One end is made totally reflecting, and the other is partially reflecting. A fraction of the light intensity passes through the partially reflecting end, forming the beam of laser light.Слайд 29Lasers
One device that exhibits stimulated emission of radiation is the
helium–neon gas laser. Figure is an energy-level diagram for the
neon atom in this system. The mixture of helium and neon is confined to a glass tube that is sealed at the ends by mirrors. A voltage applied across the tube causes electrons to sweep throughthe tube, colliding with the atoms of the gases and raising them into excited states. Neon atoms are excited to state E3* through this process (the asterisk indicates a metastable state) and also as a result of collisions with excited helium atoms. Stimulated emission occurs, causing neon atoms to make transitions to state E2. Neighboring excited atoms are also stimulated. The result is the production of coherent light at a wavelength of 632.8 nm.