Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Molecules and solids
Содержание
- 1. Molecules and solids
- 2. Molecular BondsA potential energy function that can
- 3. Molecular BondsPotential energy versus internuclear separation distance
- 4. Molecular BondsIonic BondingTotal energy versusinternuclear separation distance forNa+ and Cl- ions.
- 5. Molecular BondsCovalent BondingA covalent bond between two
- 6. Molecular BondsVan der Waals BondingYou might think
- 7. Molecular BondsVan der Waals BondingThe second type,
- 8. Molecular BondsHydrogen BondingBecause hydrogen has only one
- 9. Energy States and Spectra of MoleculesRotational Motion of Moleculesthe reduced mass of the molecule
- 10. Energy States and Spectra of MoleculesRotational Motion of MoleculesJ is the rotational quantum number
- 11. Energy States and Spectra of MoleculesRotational Motion of Molecules
- 12. Energy States and Spectra of MoleculesVibrational Motion of Moleculesv is the vibrational quantum number
- 13. Energy States and Spectra of MoleculesVibrational Motion of Molecules
- 14. Energy States and Spectra of MoleculesMolecular SpectraThe
- 15. Molecular SpectraEnergy States and Spectra of MoleculesWhen
- 16. Molecular SpectraEnergy States and Spectra of Molecules
- 17. Bonding in SolidsIonic Solidsα is a dimensionless number known as the Madelung constant
- 18. Bonding in SolidsIonic SolidsTotal potential energy versus
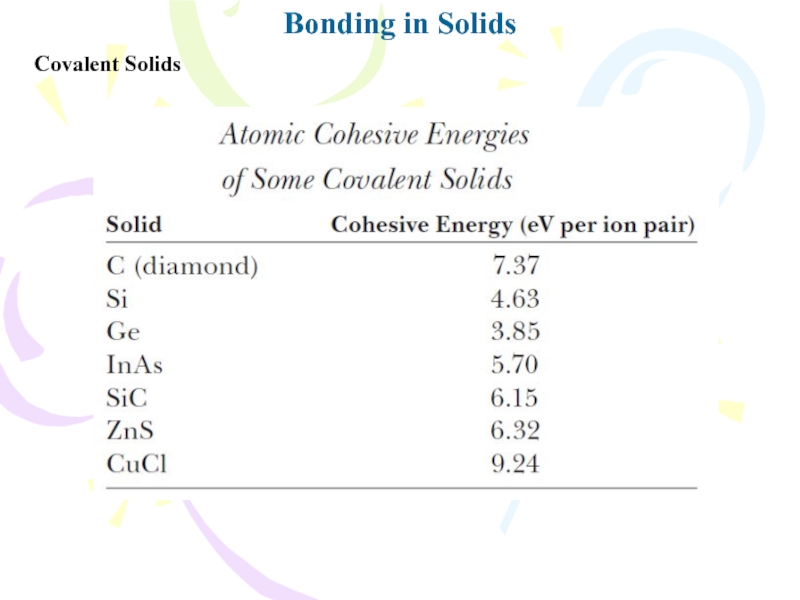
- 19. Bonding in SolidsCovalent Solids
- 20. Bonding in SolidsCovalent Solids
- 21. Bonding in SolidsMetallic SolidsMetallic bonds are generally
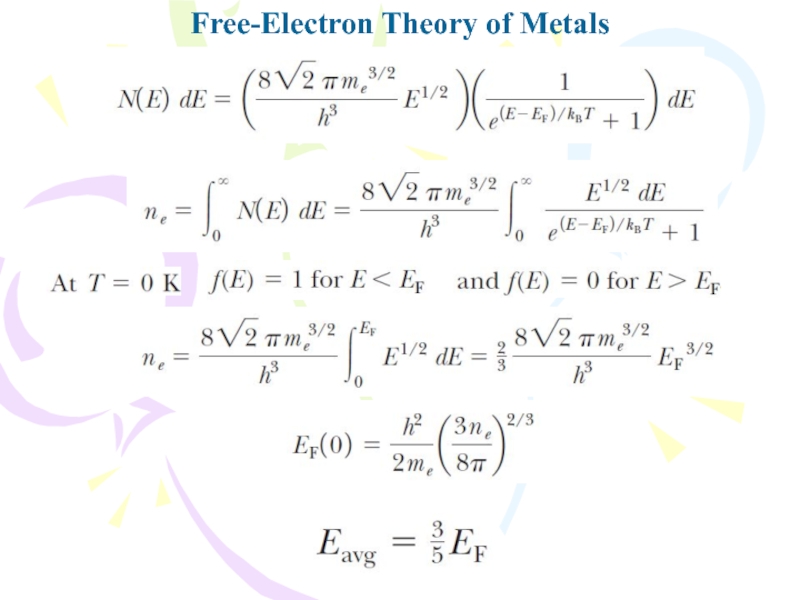
- 22. Free-Electron Theory of MetalsThe probability that a
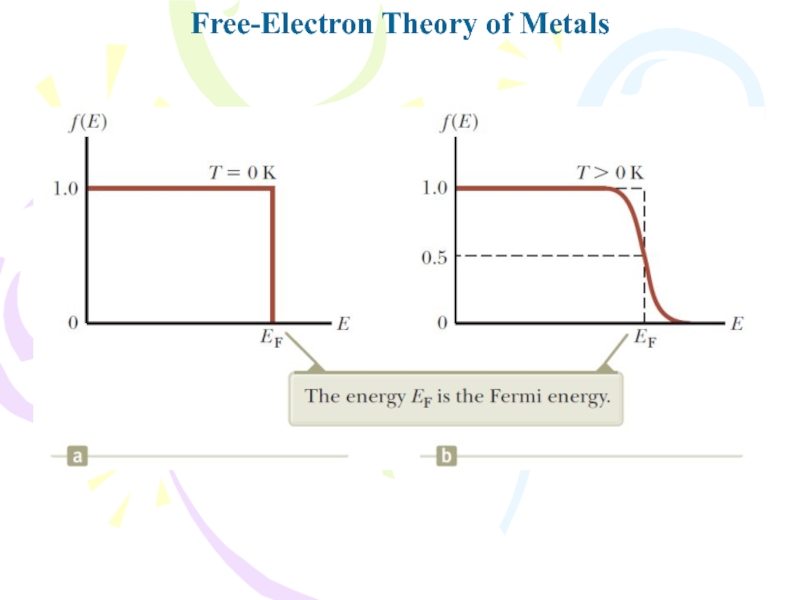
- 23. Free-Electron Theory of Metals
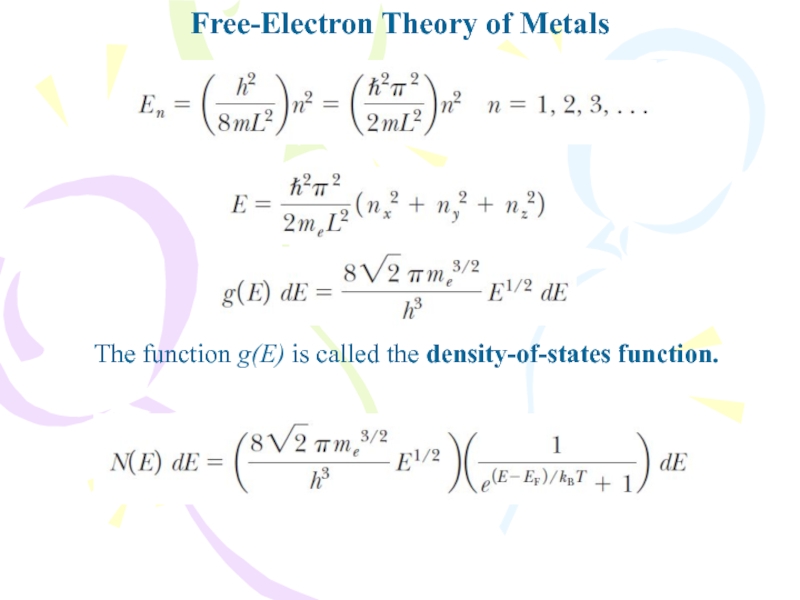
- 24. Free-Electron Theory of MetalsThe function g(E) is called the density-of-states function.
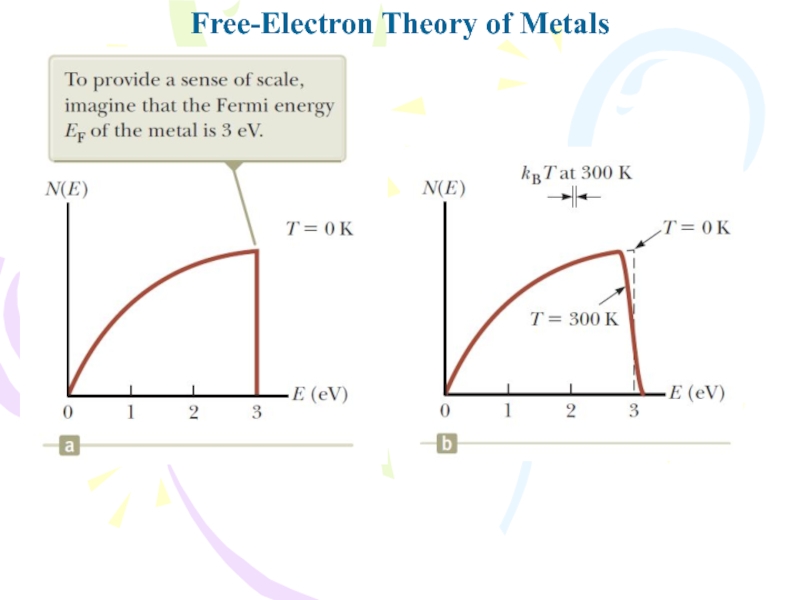
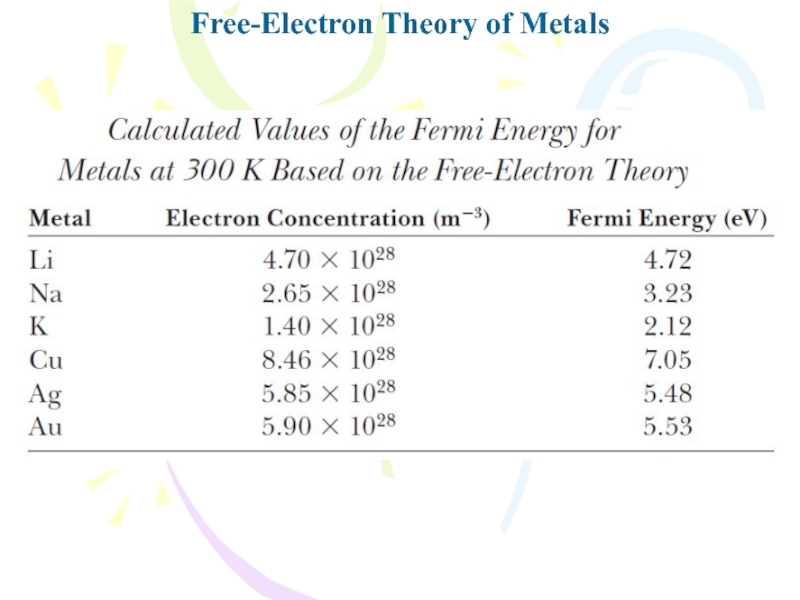
- 25. Free-Electron Theory of Metals
- 26. Free-Electron Theory of Metals
- 27. Free-Electron Theory of Metals
- 28. Band Theory of Solids
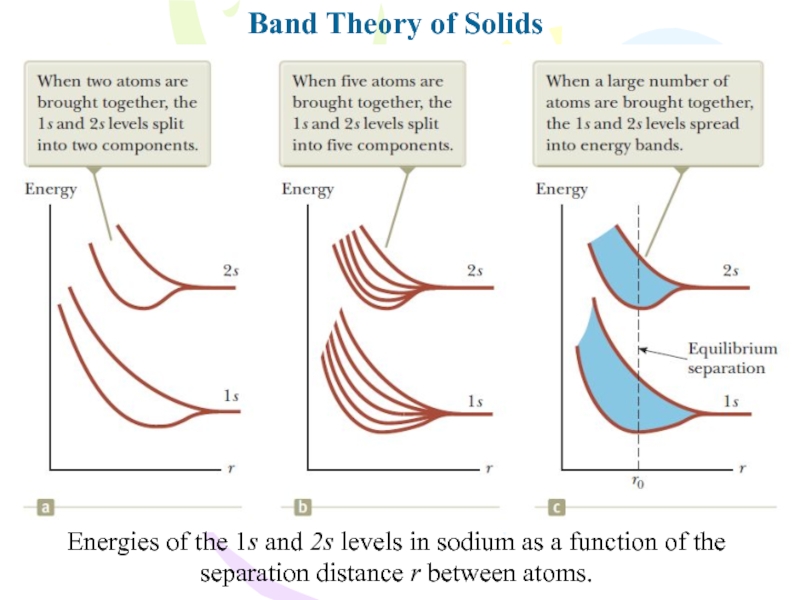
- 29. Band Theory of SolidsEnergies of the 1s
- 30. Band Theory of SolidsEnergy bands of a
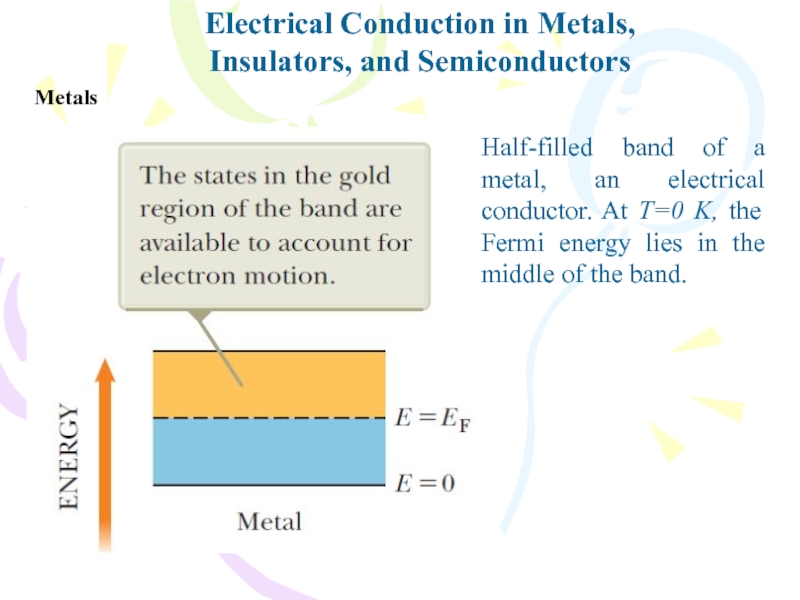
- 31. Electrical Conduction in Metals,Insulators, and SemiconductorsMetalsHalf-filled band
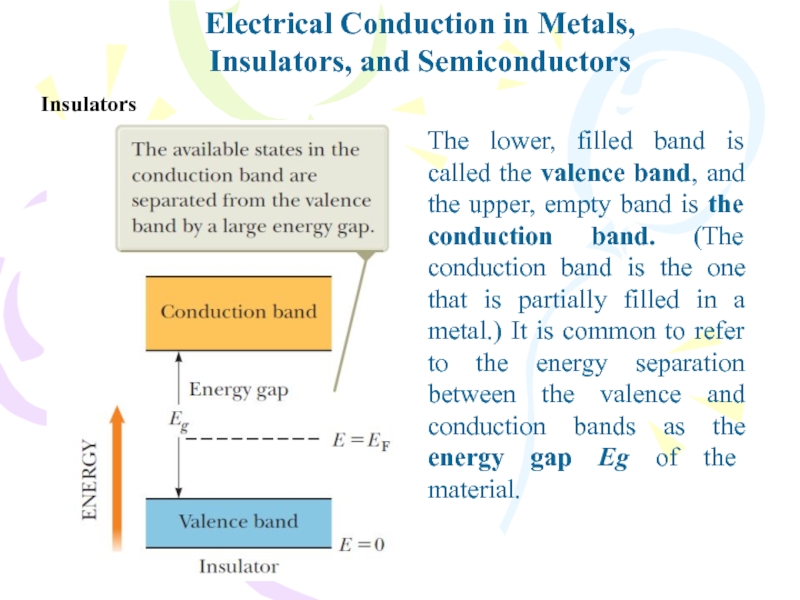
- 32. Electrical Conduction in Metals,Insulators, and SemiconductorsInsulatorsThe lower,
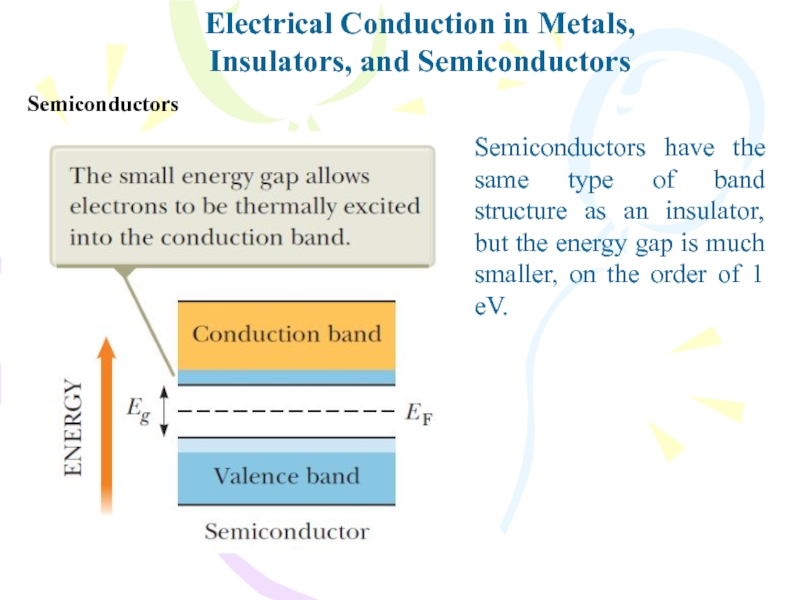
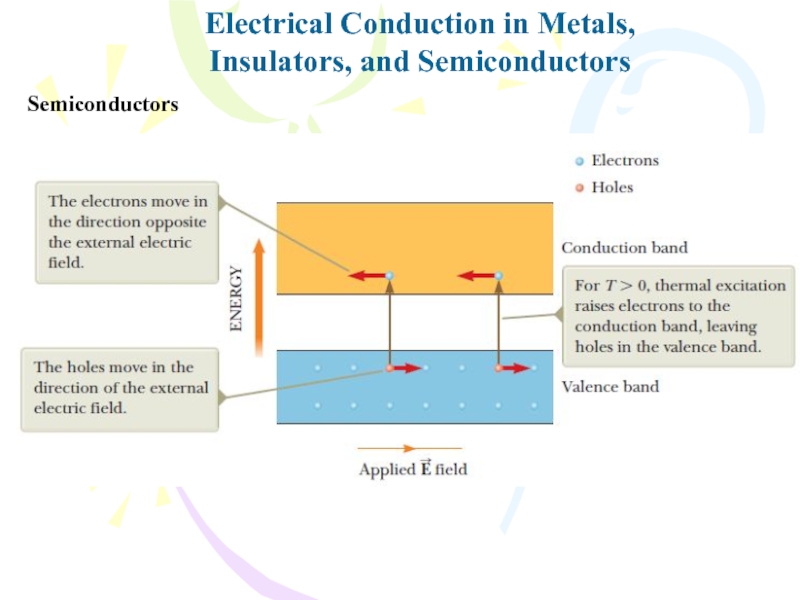
- 33. Electrical Conduction in Metals,Insulators, and SemiconductorsSemiconductorsSemiconductors have
- 34. Electrical Conduction in Metals,Insulators, and SemiconductorsSemiconductors
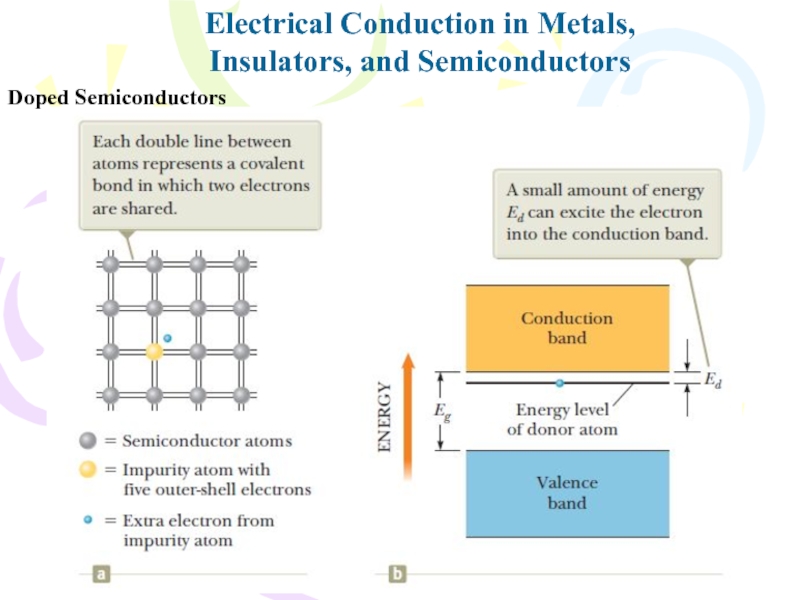
- 35. Doped SemiconductorsElectrical Conduction in Metals,Insulators, and Semiconductors
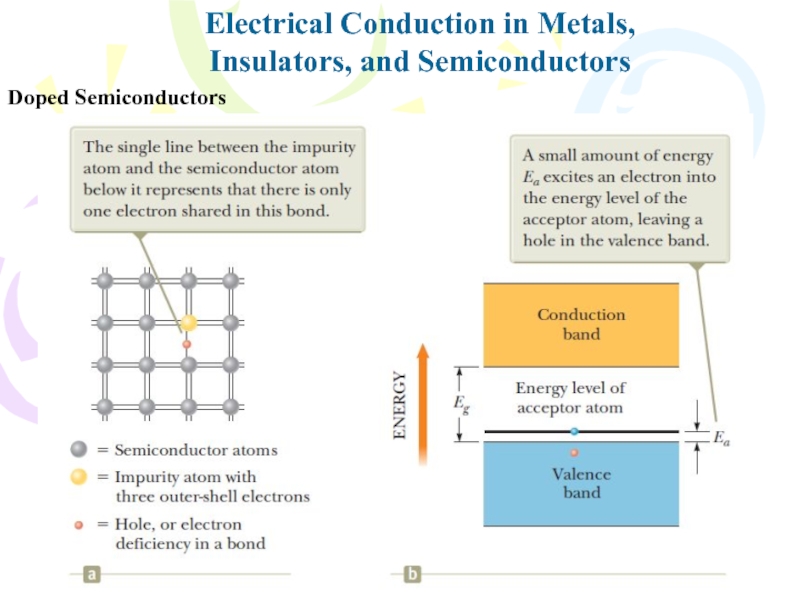
- 36. Electrical Conduction in Metals,Insulators, and SemiconductorsDoped Semiconductors
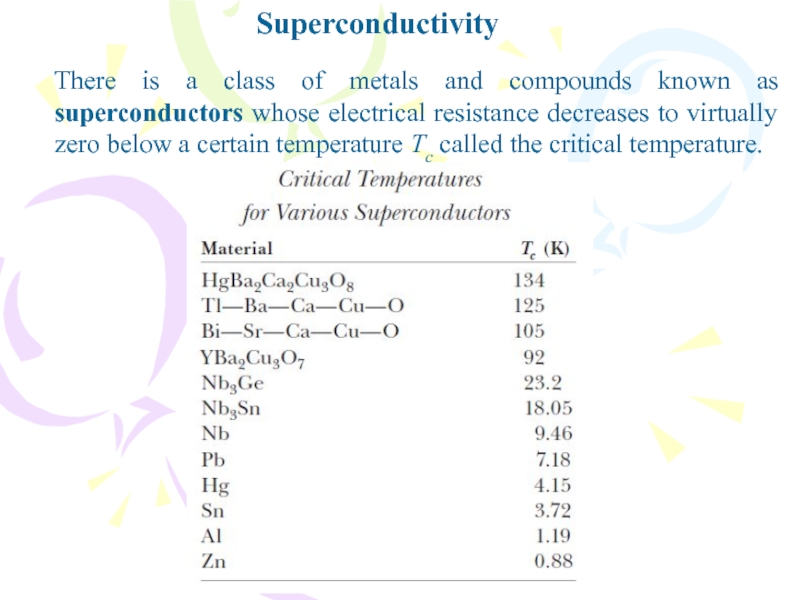
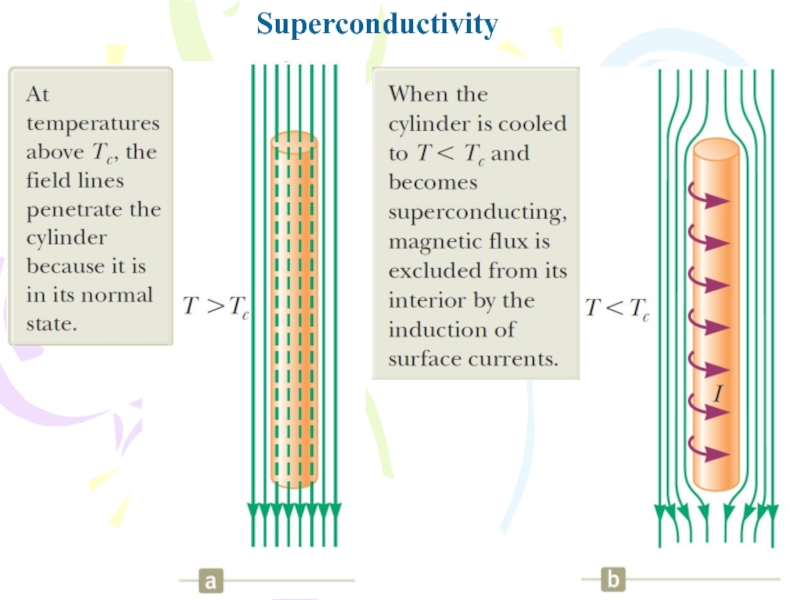
- 37. SuperconductivityThere is a class of metals and
- 38. Superconductivity
- 39. Скачать презентанцию
Слайды и текст этой презентации
Слайд 2Molecular Bonds
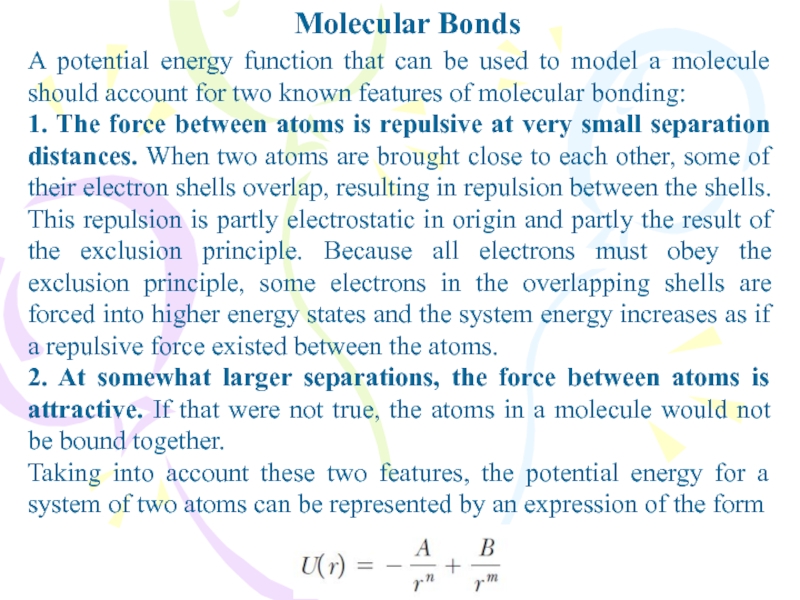
A potential energy function that can be used to
model a molecule should account for two known features of
molecular bonding:1. The force between atoms is repulsive at very small separation distances. When two atoms are brought close to each other, some of their electron shells overlap, resulting in repulsion between the shells. This repulsion is partly electrostatic in origin and partly the result of the exclusion principle. Because all electrons must obey the exclusion principle, some electrons in the overlapping shells are forced into higher energy states and the system energy increases as if a repulsive force existed between the atoms.
2. At somewhat larger separations, the force between atoms is attractive. If that were not true, the atoms in a molecule would not be bound together.
Taking into account these two features, the potential energy for a system of two atoms can be represented by an expression of the form
Слайд 3Molecular Bonds
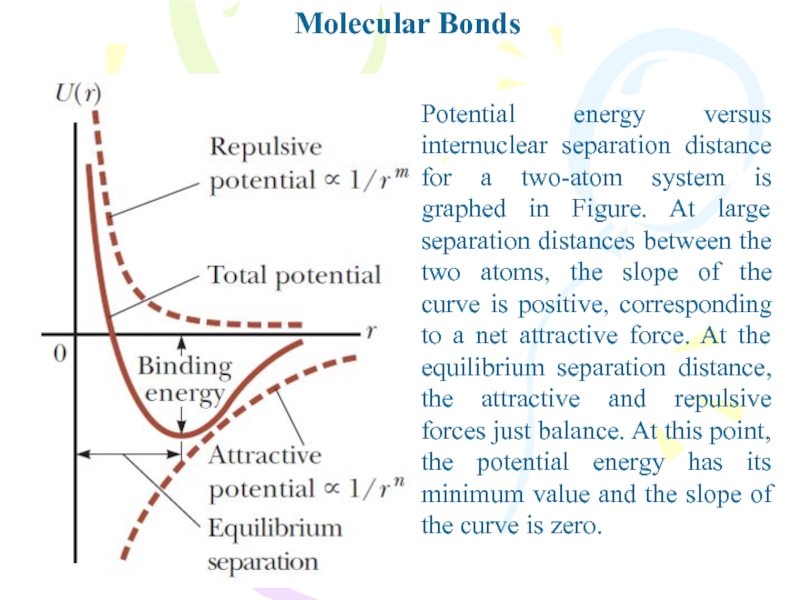
Potential energy versus internuclear separation distance for a two-atom
system is graphed in Figure. At large separation distances between
the two atoms, the slope of the curve is positive, corresponding to a net attractive force. At the equilibrium separation distance, the attractive and repulsive forces just balance. At this point, the potential energy has its minimum value and the slope of the curve is zero.Слайд 4Molecular Bonds
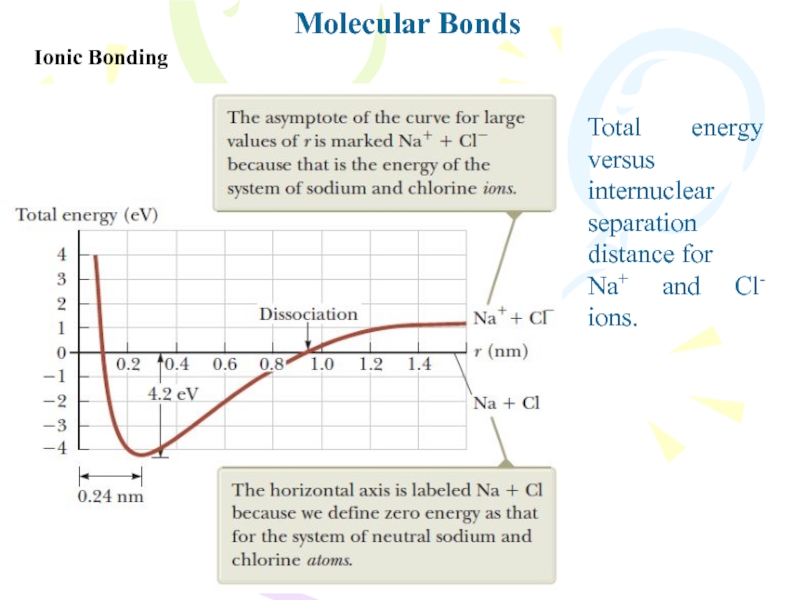
Ionic Bonding
Total energy versus
internuclear separation distance for
Na+ and Cl-
ions.
Слайд 5Molecular Bonds
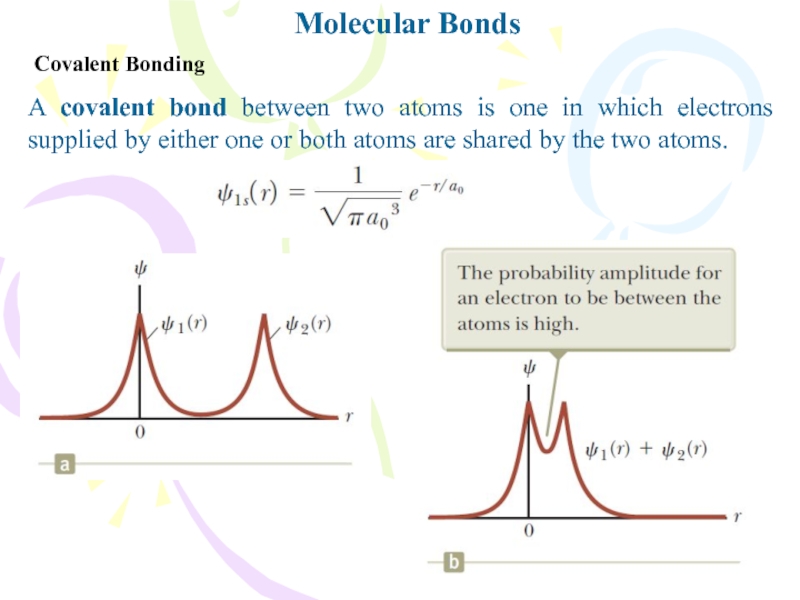
Covalent Bonding
A covalent bond between two atoms is one
in which electrons supplied by either one or both atoms
are shared by the two atoms.Слайд 6Molecular Bonds
Van der Waals Bonding
You might think that two neutral
molecules would not interact by means of the electric force
because they each have zero net charge. They are attracted to each other, however, by weak electrostatic forces called van der Waals forces.There are three types of van der Waals forces. The first type, called the dipole–dipole force, is an interaction between two molecules each having a permanent electric dipole moment. For example, polar molecules such as HCl have permanent electric dipole moments and attract other polar molecules.
Слайд 7Molecular Bonds
Van der Waals Bonding
The second type, the dipole–induced dipole
force, results when a polar molecule having a permanent electric
dipole moment induces a dipole moment in a nonpolar molecule. In this case, the electric field of the polar molecule creates the dipole moment in the nonpolar molecule, which then results in an attractive force between the molecules.The third type is called the dispersion force, an attractive force that occurs between two nonpolar molecules. In this case, although the average dipole moment of a nonpolar molecule is zero, the average of the square of the dipole moment is nonzero because of charge fluctuations. Two nonpolar molecules near each other tend to have dipole moments that are correlated in time so as to produce an attractive van der Waals force.
Слайд 8Molecular Bonds
Hydrogen Bonding
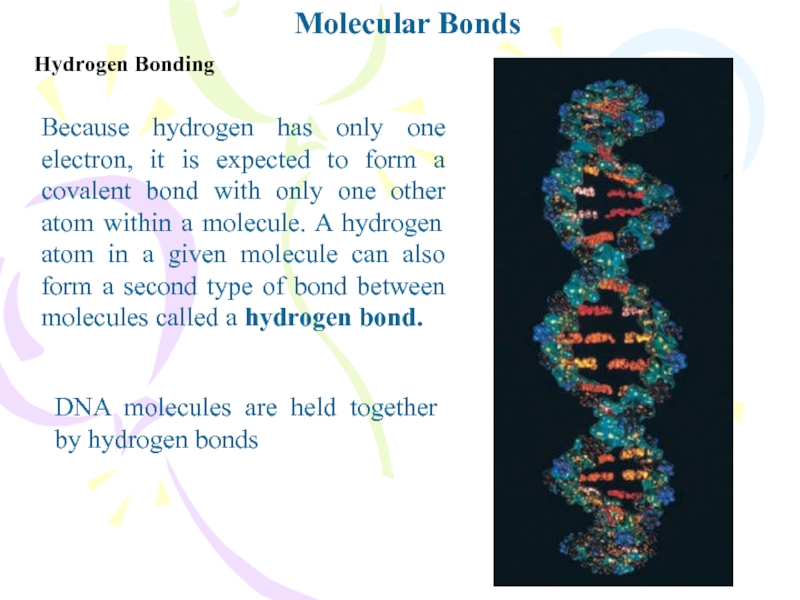
Because hydrogen has only one electron, it is
expected to form a covalent bond with only one other
atom within a molecule. A hydrogen atom in a given molecule can also form a second type of bond between molecules called a hydrogen bond.DNA molecules are held together by hydrogen bonds
Слайд 9Energy States and Spectra of Molecules
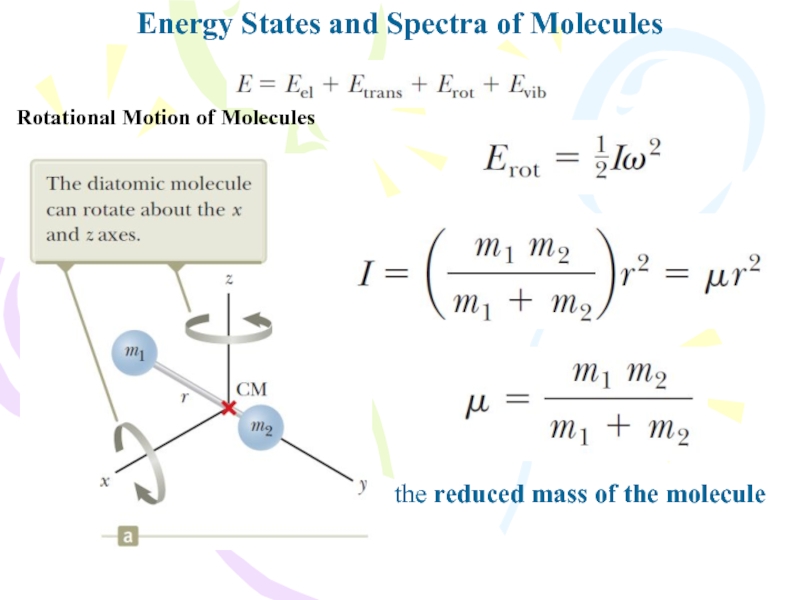
Rotational Motion of Molecules
the reduced
mass of the molecule
Слайд 10Energy States and Spectra of Molecules
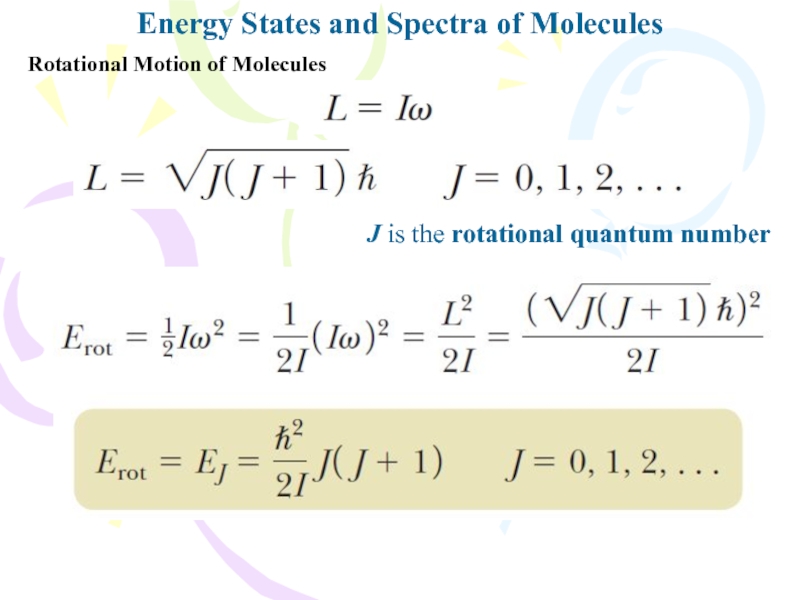
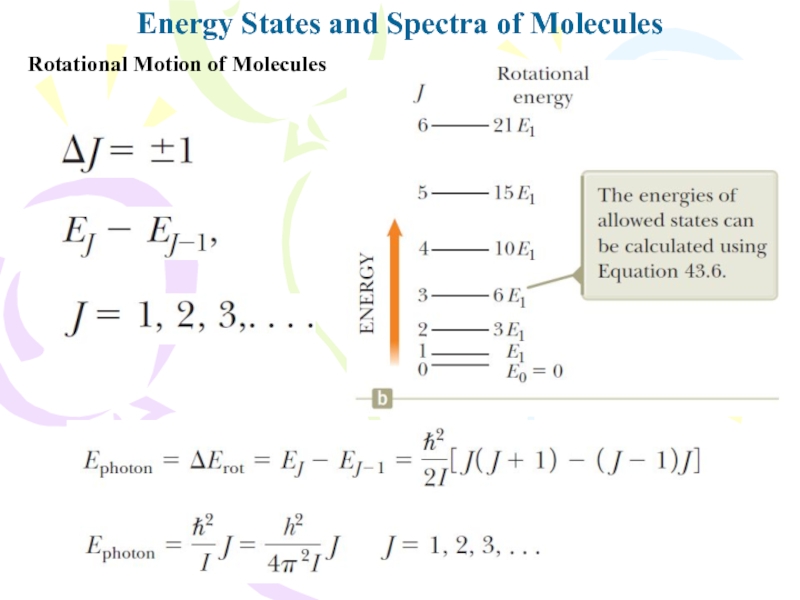
Rotational Motion of Molecules
J is
the rotational quantum number
Слайд 12Energy States and Spectra of Molecules
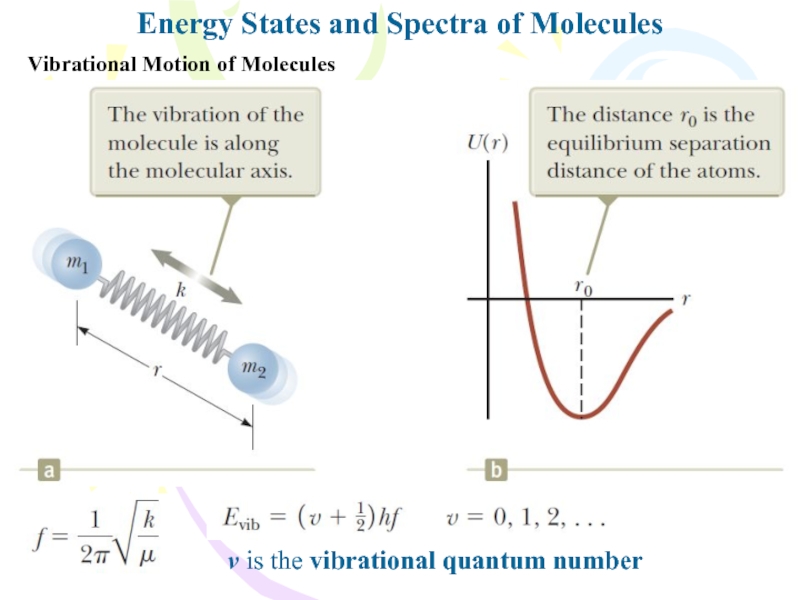
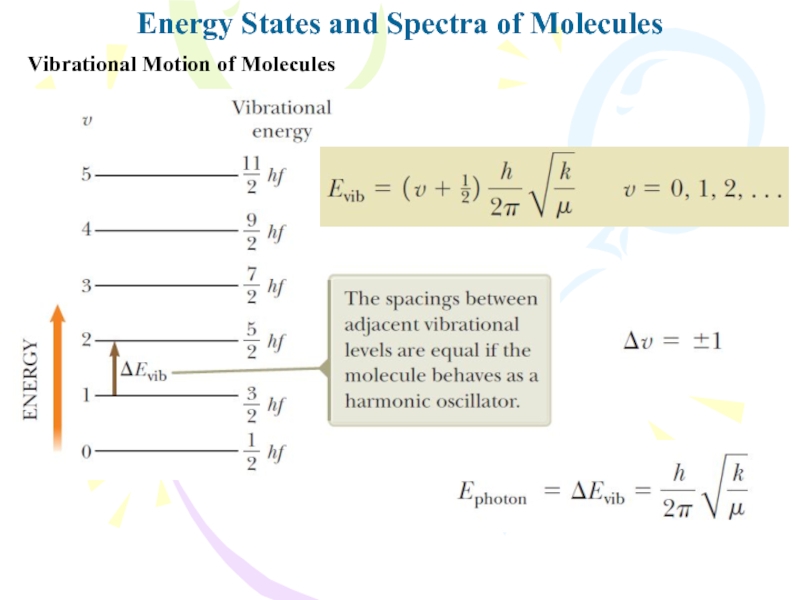
Vibrational Motion of Molecules
v is
the vibrational quantum number
Слайд 14Energy States and Spectra of Molecules
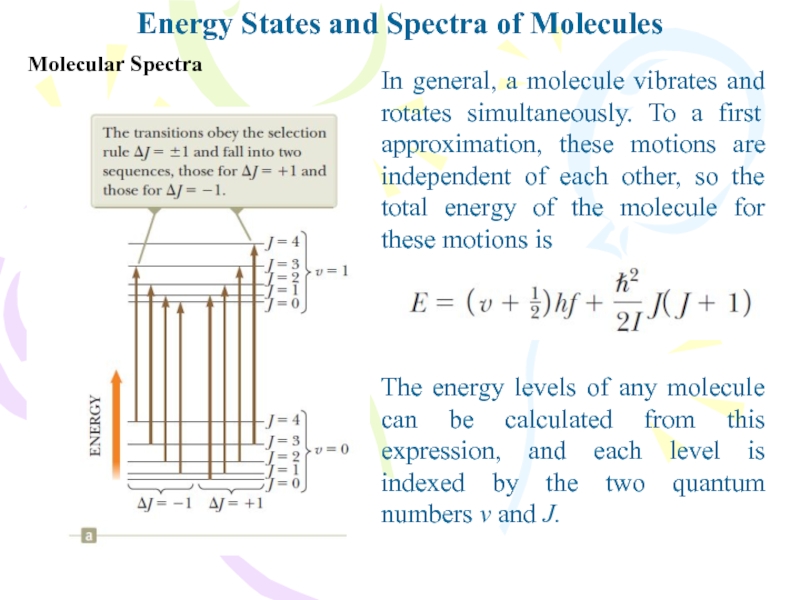
Molecular Spectra
The energy levels of
any molecule can be calculated from this expression, and each
level is indexed by the two quantum numbers v and J.In general, a molecule vibrates and rotates simultaneously. To a first approximation, these motions are independent of each other, so the total energy of the molecule for these motions is
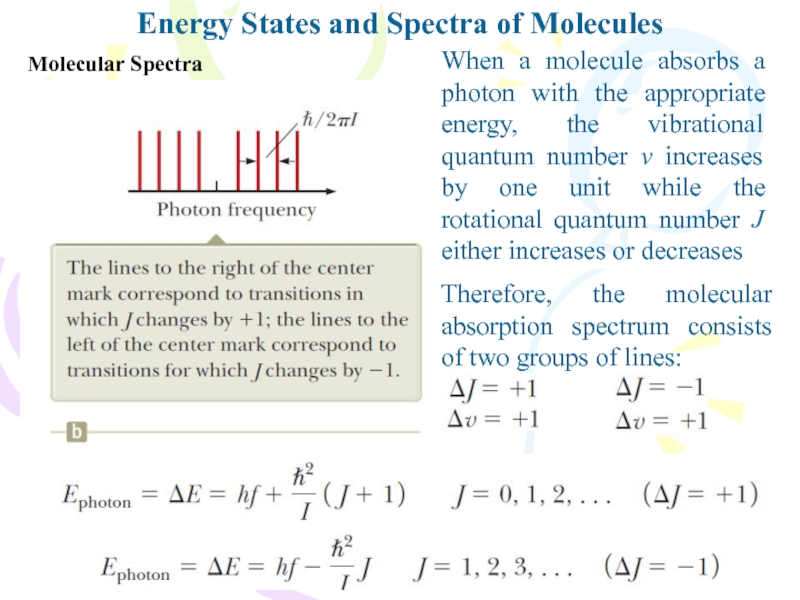
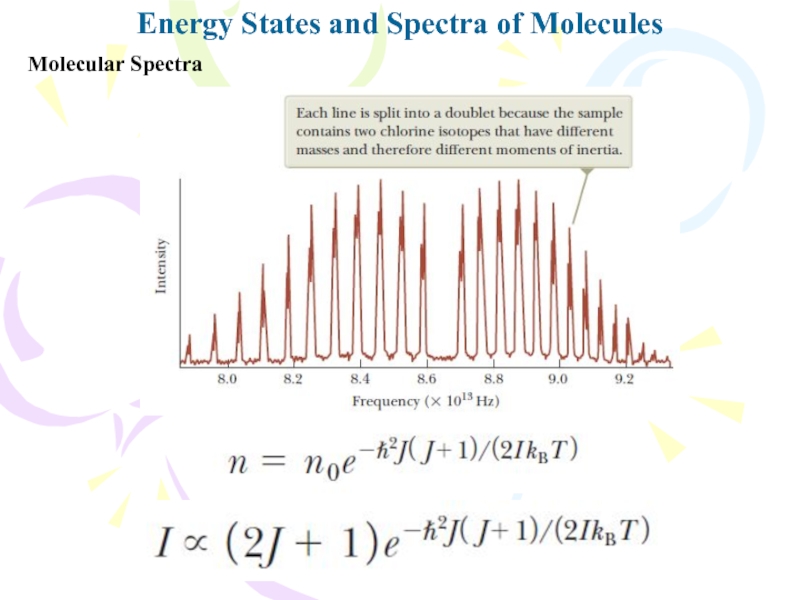
Слайд 15Molecular Spectra
Energy States and Spectra of Molecules
When a molecule absorbs
a photon with the appropriate energy, the vibrational quantum number
v increases by one unit while the rotational quantum number J either increases or decreasesTherefore, the molecular absorption spectrum consists of two groups of lines:
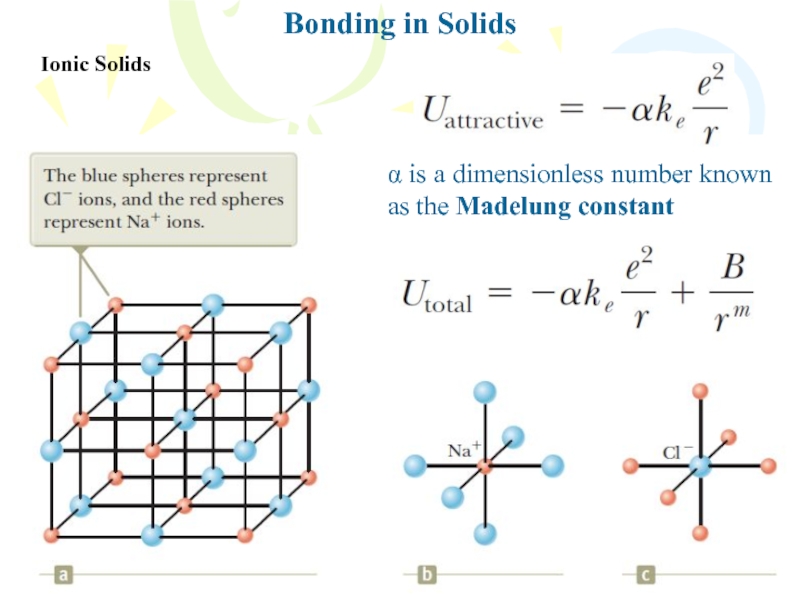
Слайд 18Bonding in Solids
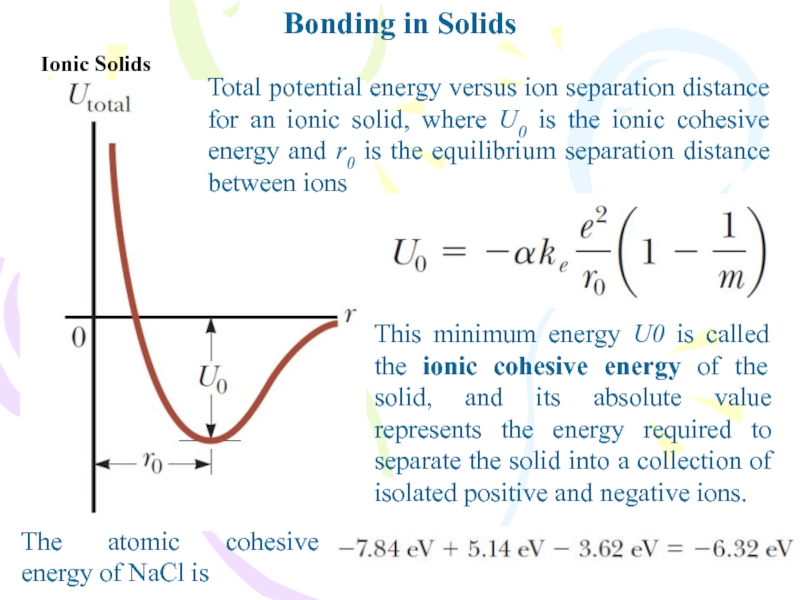
Ionic Solids
Total potential energy versus ion separation distance
for an ionic solid, where U0 is the ionic cohesive
energy and r0 is the equilibrium separation distance between ionsThis minimum energy U0 is called the ionic cohesive energy of the solid, and its absolute value represents the energy required to separate the solid into a collection of isolated positive and negative ions.
The atomic cohesive energy of NaCl is
Слайд 21Bonding in Solids
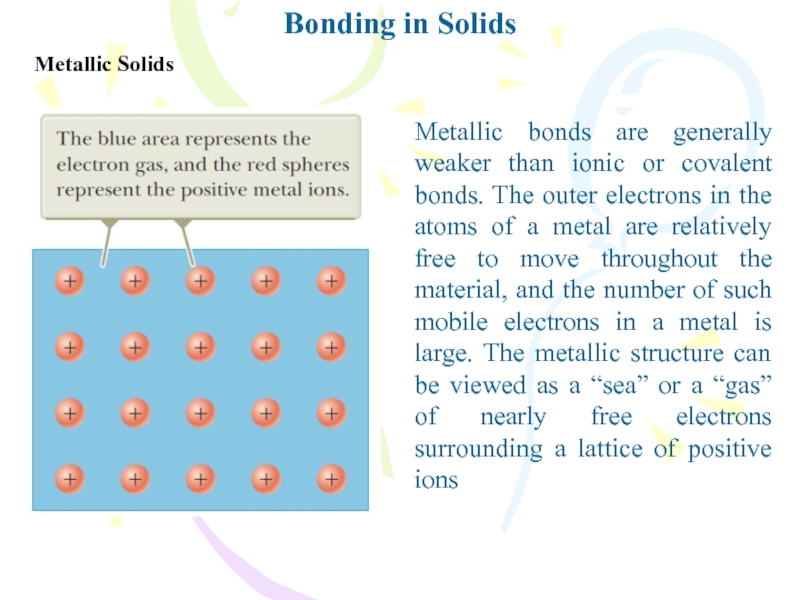
Metallic Solids
Metallic bonds are generally weaker than ionic
or covalent bonds. The outer electrons in the atoms of
a metal are relatively free to move throughout the material, and the number of such mobile electrons in a metal is large. The metallic structure can be viewed as a “sea” or a “gas” of nearly free electrons surrounding a lattice of positive ionsСлайд 22Free-Electron Theory of Metals
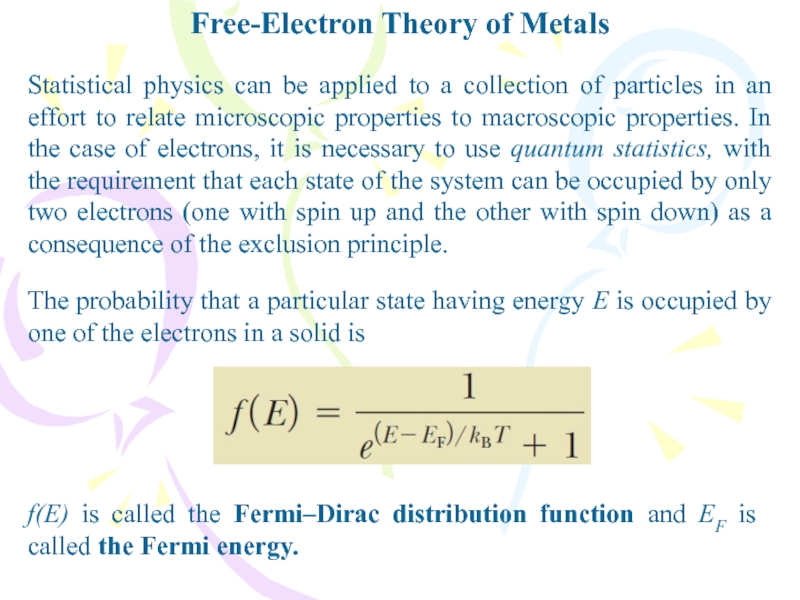
The probability that a particular state having
energy E is occupied by one of the electrons in
a solid isf(E) is called the Fermi–Dirac distribution function and EF is called the Fermi energy.
Statistical physics can be applied to a collection of particles in an effort to relate microscopic properties to macroscopic properties. In the case of electrons, it is necessary to use quantum statistics, with the requirement that each state of the system can be occupied by only two electrons (one with spin up and the other with spin down) as a consequence of the exclusion principle.
Слайд 29Band Theory of Solids
Energies of the 1s and 2s levels
in sodium as a function of the separation distance r
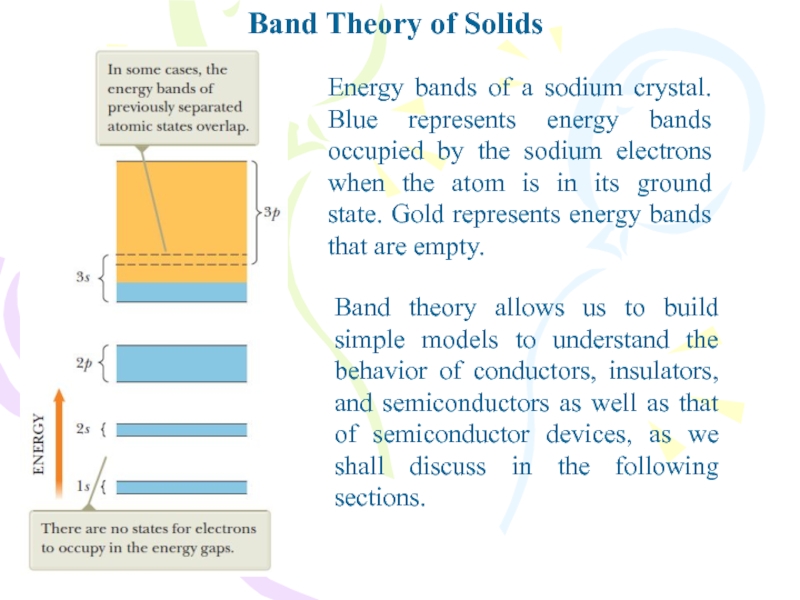
between atoms.Слайд 30Band Theory of Solids
Energy bands of a sodium crystal. Blue
represents energy bands occupied by the sodium electrons when the
atom is in its ground state. Gold represents energy bands that are empty.Band theory allows us to build simple models to understand the behavior of conductors, insulators, and semiconductors as well as that of semiconductor devices, as we shall discuss in the following sections.