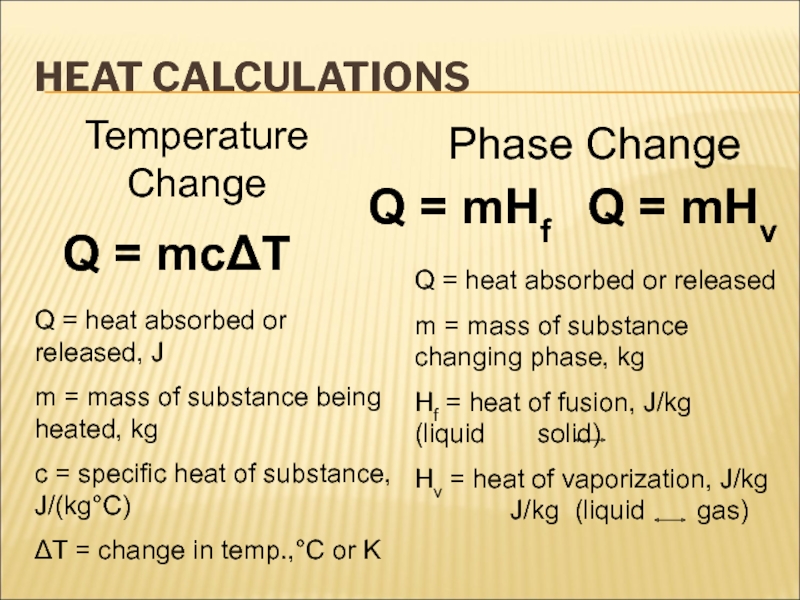
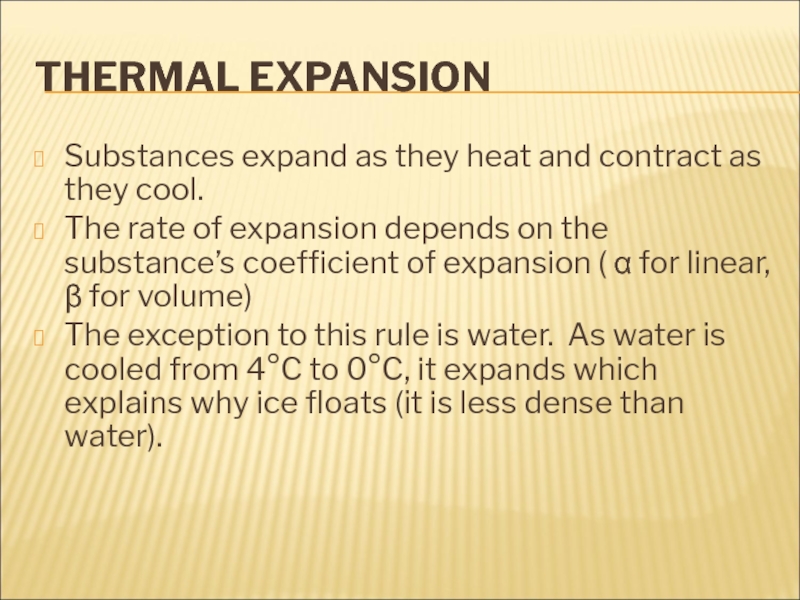
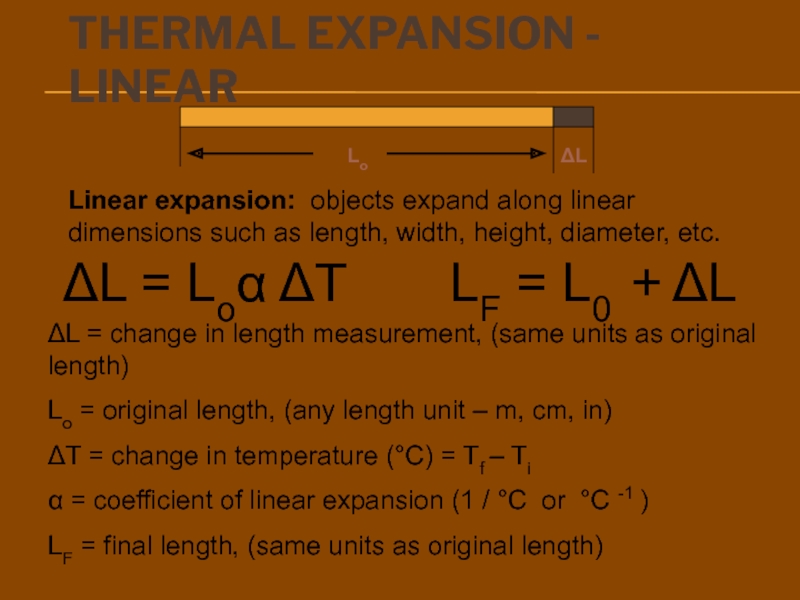
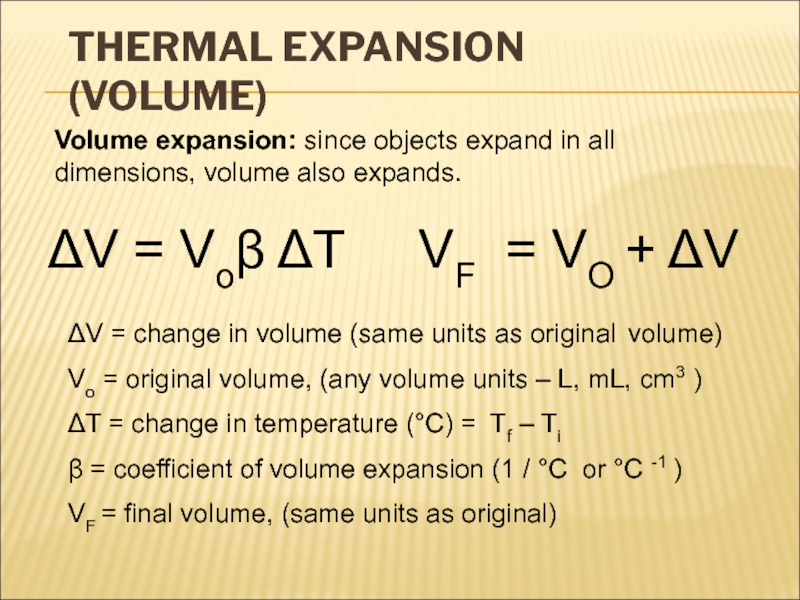
There is no “coldness” energy
Any object with temperature above zero
Kelvin has heat energyTemperature
Avg. Kinetic Energy of the particles
Measured in C, F, K
“hot” & “cold are relative terms
Absolute zero is zero Kelvin