Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Human Tyrosyl-DNA Phosphodiesterase 1 : new activities and development of
Содержание
- 1. Human Tyrosyl-DNA Phosphodiesterase 1 : new activities and development of
- 2. -AlkylatingagentsO6-meGdirect repair- UV-light- Environmental mutagens pyrimidine dimersbulky
- 3. Defects in DNA repair systemsCancerAgingCataractProgressivecerebralpalsyIntellectualand immuneinadequacyCockayne’s syndromXeroderma pigmentosum(melanoma,carcinoma)Trichothio-distrophy
- 4. DNA repair systems suppress the efficiency
- 5. Surface representation of the crystal structures for
- 6. Defects in step catalysed by TDP1 causes
- 7. Topoisomerase1 ( Top1) mediated DNA relaxation by controlled rotationControlled rotationRelaxation of supercoilingReligationSupercoiled duplexNickingSupercoiled DNARelaxed DNA
- 8. DNA lesionsEndogenousExogenousabasic sites8-oxoguanosine5-hydroxycytosinecytosine methylationphoto dimersO6-methylguanineN6-ethenoadenineN2-dG-benzo[a]pyreneadduct
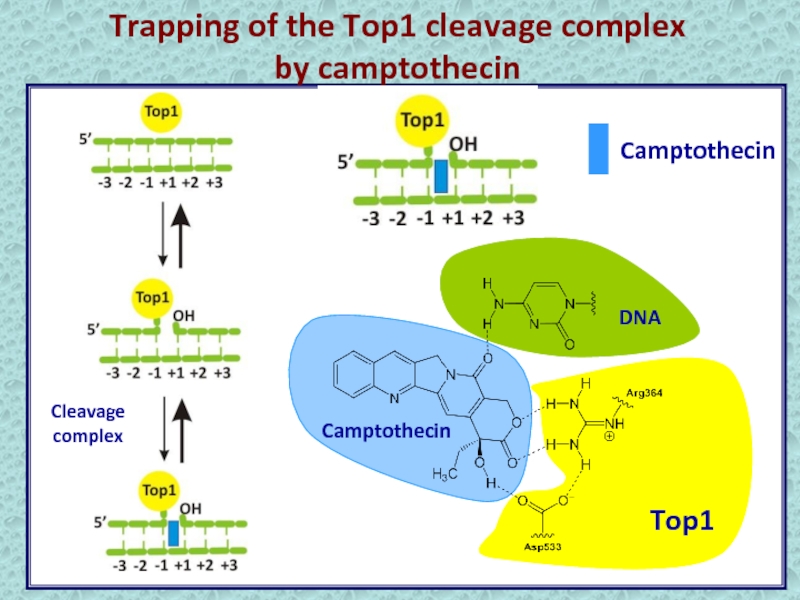
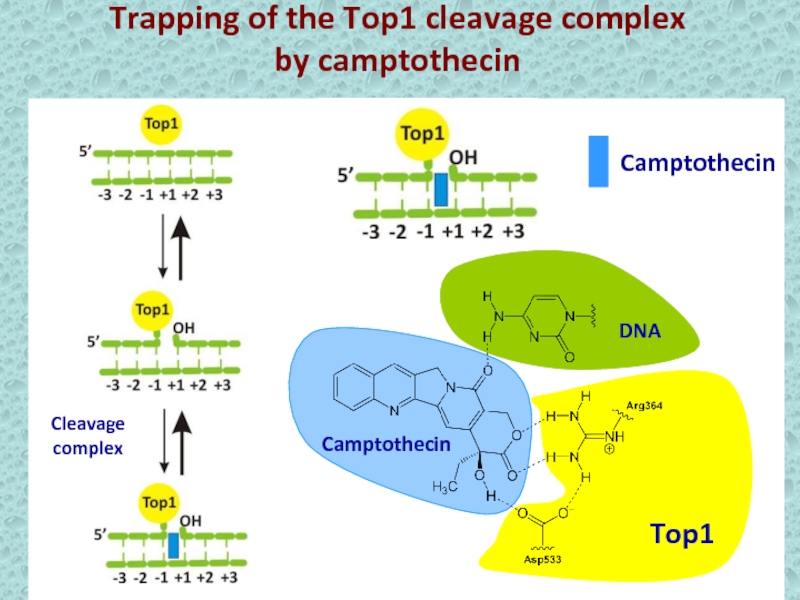
- 9. Trapping of the Top1 cleavage complexby camptothecinCamptothecinCleavage complex
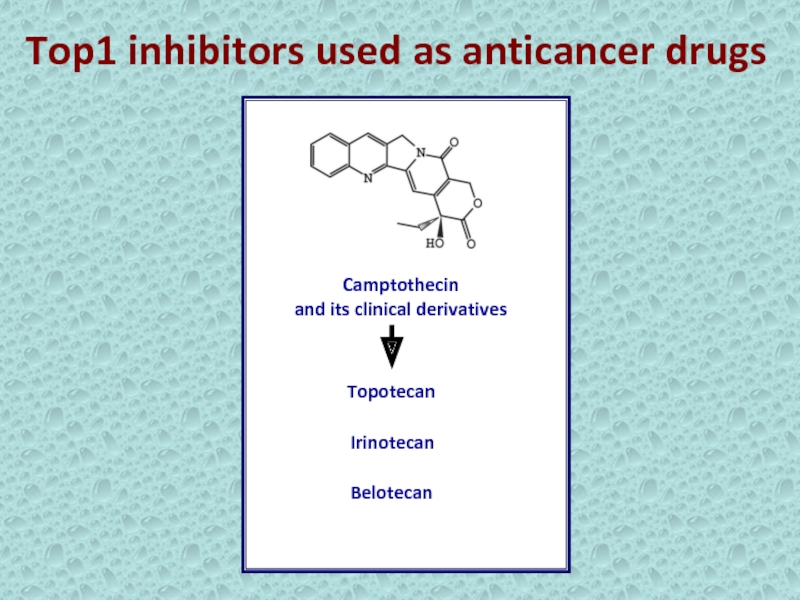
- 10. Top1 inhibitors used as anticancer drugs Camptothecin and its clinical derivativesTopotecanIrinotecanBelotecan
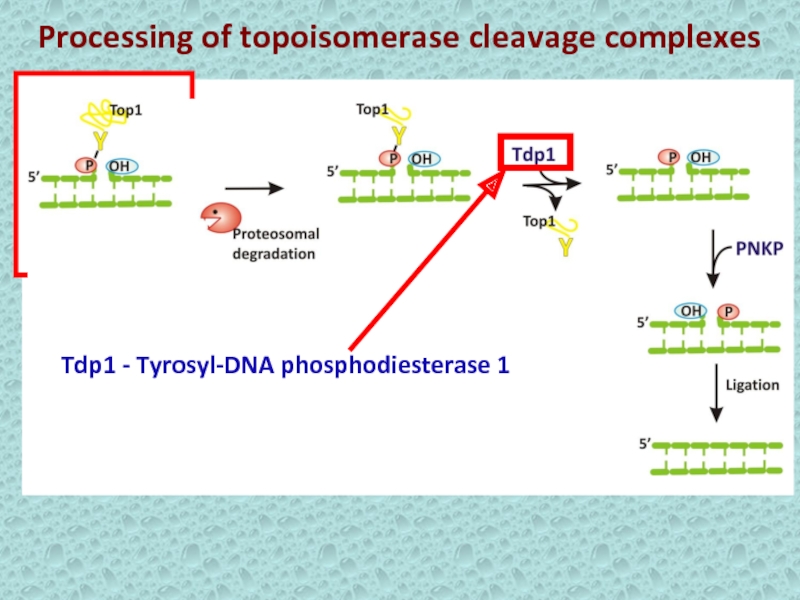
- 11. Processing of topoisomerase cleavage complexes Tdp1 - Tyrosyl-DNA phosphodiesterase 1
- 12. Trapping of the Top1 cleavage complexby camptothecinCamptothecinCleavage complex
- 13. Tdp1 is responsible for the resistance
- 14. Therapeutic agents causing hypersensitivity of Tdp1-deficient cells
- 15. TDP1 processes DNA adducts of Top1 and
- 16. АР sites appeared in DNA at an
- 17. TDP1 catalyzes the cleavage of integral AP
- 18. Tyrosyl-DNA phosphodiesterase 1 initiates repair of AP
- 19. Tyrosyl-DNA phosphodiesterase 1 is a component of
- 20. Defects in step catalysed by TDP1 causes
- 21. Tdp1 catalytic cycle
- 22. Vanadate and tungstateinhibit Tdp1 at millimolar concentrations,
- 23. Natural compound extracted from marine ascidian Varacin
- 24. Real-time detection of TDP1 activityZakharenko A., et
- 25. Several novel benzopentathiepines were synthesized and tested
- 26. Molecular Modeling of the most potent TDP1
- 27. - The cytotoxicity study of the compounds
- 28. The X-ray structure of TDP1 (D148) Conclusions-
- 29. PARP1 is important in maintaining telomere length
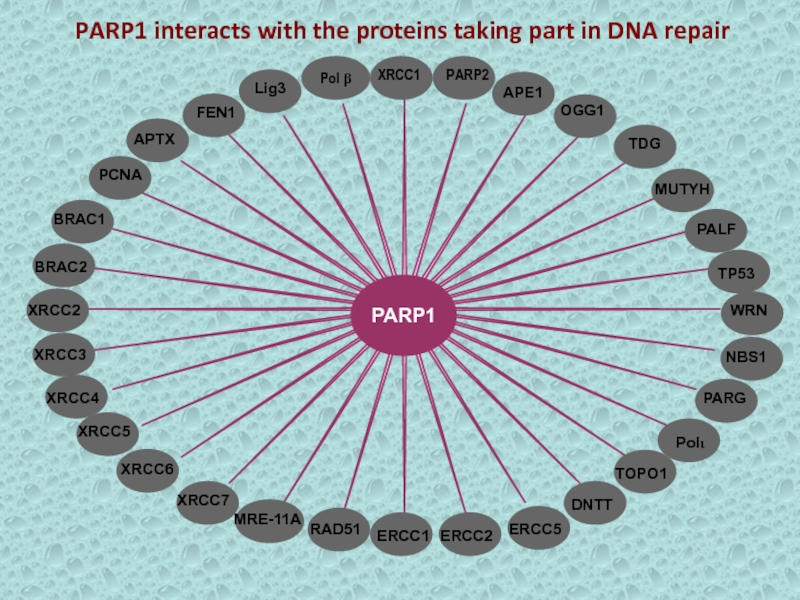
- 30. FEN1APE1PALFOGG1Lig3APTXPCNAMUTYHTDGBRAC1BRAC2XRCC2XRCC3XRCC4XRCC5XRCC6XRCC7RAD51MRE-11ATP53ERCC1ERCC2ERCC5NBS1WRNPARGPoliDNTTTOPO1 PARP1 interacts with the proteins taking part in DNA repair
- 31. NAD and ATP store depletion Neurodegenerative diseasesStrokeCraniocerebral
- 32. Lichen, marine ascidian, licorice and birch overcome
- 33. Our teamN.F. SalakhutdinovHead of Department of medical
- 34. Thank you for your attention!
- 35. Therapeutic agents causing hypersensitivity of Tdp1-deficient cells
- 36. Top1 inhibitorsCamptothecin and its clinical derivativesTopotecanirinotecanBelotecanTopovaleArc-111Indenoisoquinolines
- 37. The increment of FITC -labeled annexin V
- 38. Tdp1 inhibitorsAminoglycoside antibiotics and ribosome inhibitors, common
- 39. Скачать презентанцию
Слайды и текст этой презентации
Слайд 1Human Tyrosyl-DNA Phosphodiesterase 1 : new
activities and development of enzyme
inhibitors as anticancer drugs
and Fundamental Medicine, Department of Physicochemical Biology and Biotechnology of ASU, Novosibirsk, Barnaul,RussiaСлайд 2-Alkylating
agents
O6-meG
direct repair
- UV-light
- Environmental
mutagens
pyrimidine dimers
bulky adducts
nucleotide excision
repair (NER)
- X-rays
double strand
breaks
homologous
recombination (HR)
non-homologous
end joining (NHEJ)
- Replication
errors
base
mismatchesinsertions
deletions
mismatch
repair (MMR)
Most common DNA-damaging agents, lesions, and repair pathways
abasic sites
oxidized, deaminated,
alkylated bases
ssDNA breaks
base excision
repair (BER)
- Spontaneous reactions
- Oxygen radicals
- Alkylating agents
- X-rays
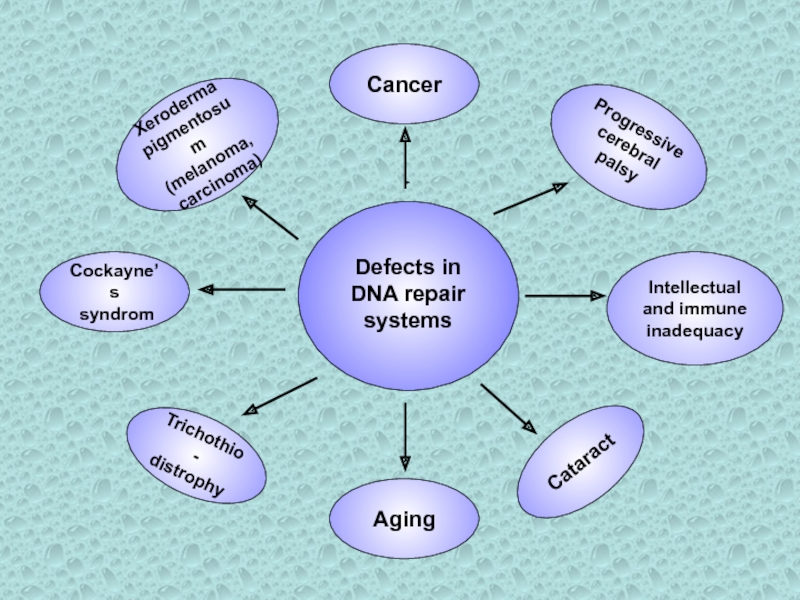
Слайд 3Defects in
DNA repair systems
Cancer
Aging
Cataract
Progressive
cerebral
palsy
Intellectual
and immune
inadequacy
Cockayne’s
syndrom
Xeroderma
pigmentosum
(melanoma,
carcinoma)
Trichothio-
distrophy
Слайд 4 DNA repair systems suppress the efficiency of a number
of antitumor drugs that have to reveal their cytotoxic effects
by damaging DNA of the cancer cells.Therefore inhibiting of DNA repair can improve the anticancer treatment.
DNA repair systems are targets for development of anticancer drugs
Слайд 5Surface representation of the crystal structures for TDP1 (PDB ID
1NOP).
Protein is represented in light pink, catalytic residues in
yellow, DNA in blue sticks, peptide in green sticks and both sticks colored by element (N, blue; O, red; P, orange; Vanadate, grey). The crystal structure of Tyrosyl-DNA Phosphodiesterase 1 (TDP1)
Detailed contacts between substrate and TDP1 residues in the catalytic site.
Catalytic residues are represented as yellow sticks; residues involved in polar interactions are in cyan sticks; residues involved in hydrophobic interactions in magenta sticks.
All stick sare colored by element (N, blue; O, red; P, orange; Vanadate, grey). Dashed lines highlight polar interactions.
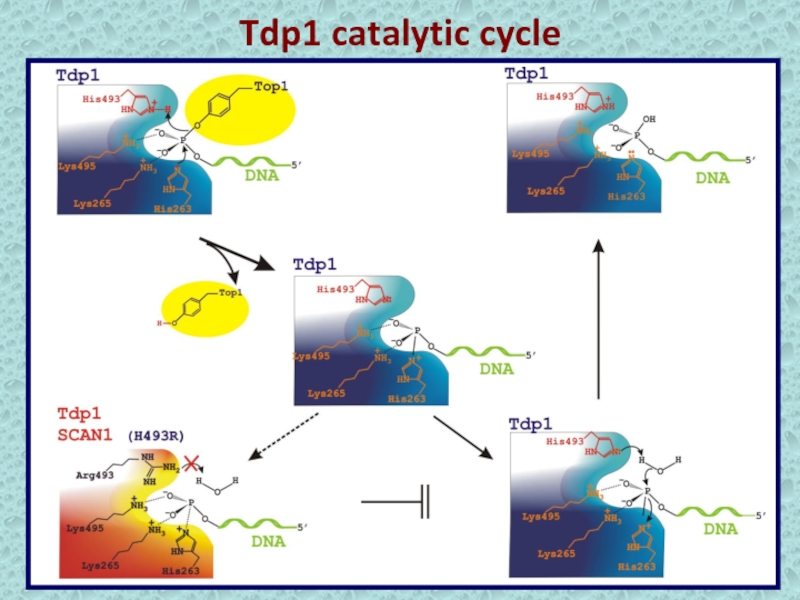
Слайд 6Defects in step catalysed by TDP1 causes cerebellar degeneration and
peripheral neuropathy typified by patients with SCAN1
Tdp1
Removal of the Top1
- DNA adductsPrevention of the anticancer drug action
H493R mutation in TDP1 is responsible for the inherited disorder, spinocerebellar ataxia with axonal neuropathy (SCAN1)
Wild
type
SCAN1 mutation
+
The cerebellum is a common target for disorders with DNA repair defects
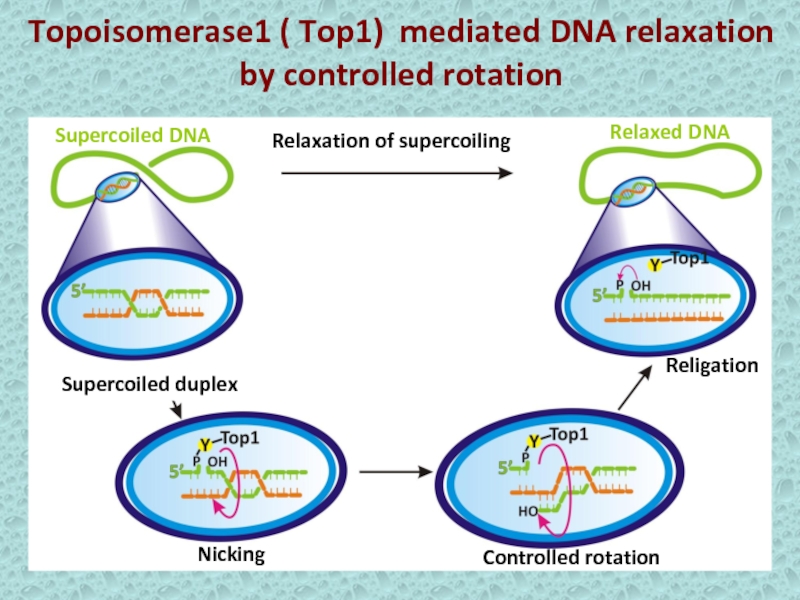
Слайд 7Topoisomerase1 ( Top1) mediated DNA relaxation
by controlled rotation
Controlled rotation
Relaxation
of supercoiling
Religation
Supercoiled duplex
Nicking
Supercoiled DNA
Relaxed DNA
Слайд 8DNA lesions
Endogenous
Exogenous
abasic sites
8-oxoguanosine
5-hydroxycytosine
cytosine methylation
photo dimers
O6-methylguanine
N6-ethenoadenine
N2-dG-benzo[a]pyrene
adduct
Слайд 10Top1 inhibitors used as anticancer drugs
Camptothecin
and its clinical
derivatives
Topotecan
Irinotecan
Belotecan
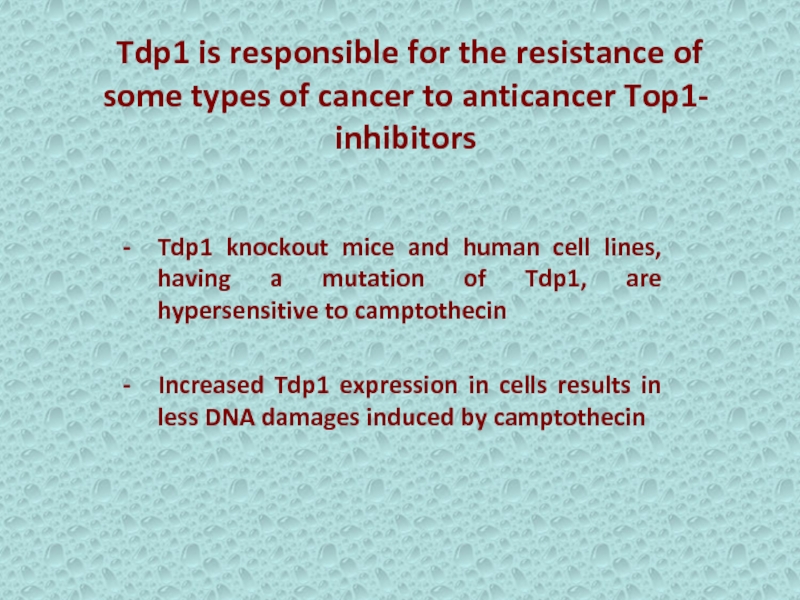
Слайд 13 Tdp1 is responsible for the resistance of some types
of cancer to anticancer Top1- inhibitors
Tdp1 knockout mice and human
cell lines, having a mutation of Tdp1, are hypersensitive to camptothecinIncreased Tdp1 expression in cells results in less DNA damages induced by camptothecin
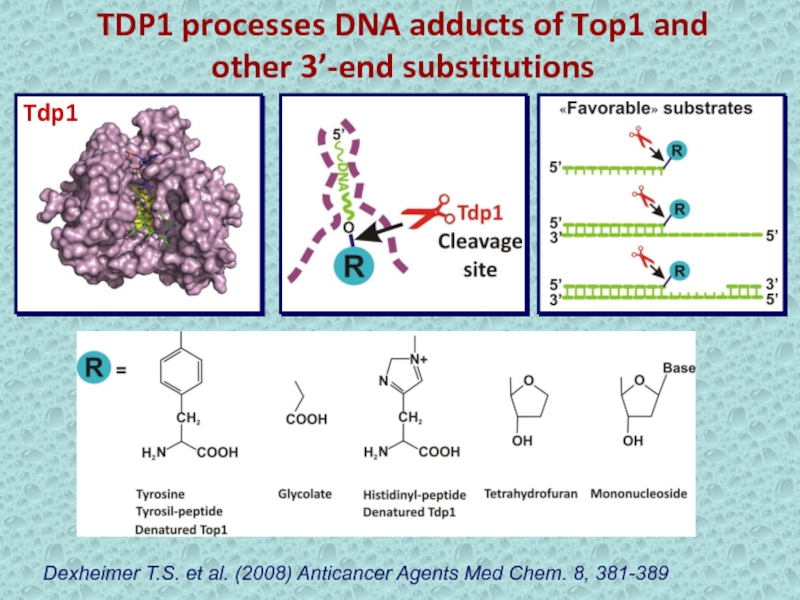
Слайд 15TDP1 processes DNA adducts of Top1 and other 3’-end substitutions
Dexheimer
T.S. et al. (2008) Anticancer Agents Med Chem. 8, 381-389
Tdp1
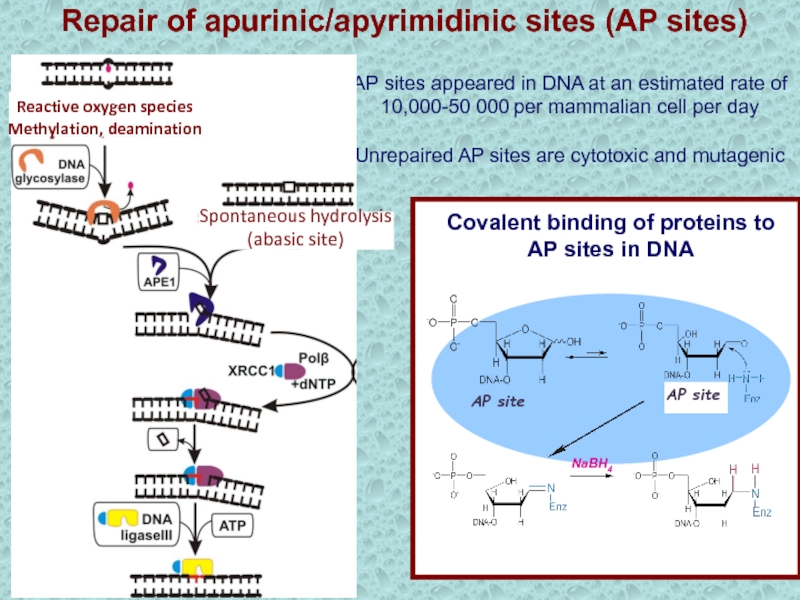
Слайд 16АР sites appeared in DNA at an estimated rate of
10,000-50 000 per mammalian cell per day
Unrepaired AP sites are
cytotoxic and mutagenicNaBH4
АР site
АР site
Covalent binding of proteins to
AP sites in DNA
Spontaneous hydrolysis
(abasic site)
Reactive oxygen species
Methylation, deamination
Repair of apurinic/apyrimidinic sites (AP sites)
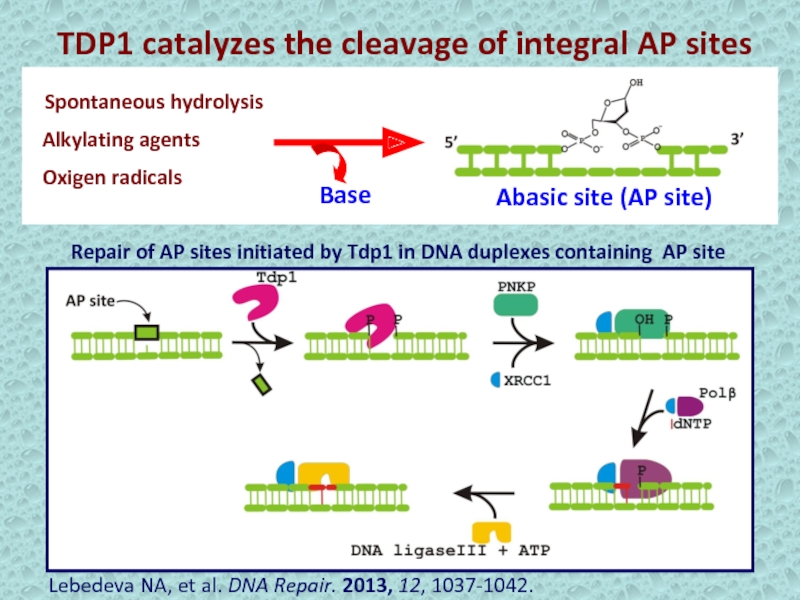
Слайд 17TDP1 catalyzes the cleavage of integral AP sites
Alkylating agents
Spontaneous hydrolysis
Oxigen
radicals
Abasic site (AP site)
Repair of AP sites initiated by
Tdp1 in DNA duplexes containing AP site Base
Lebedeva NA, et al. DNA Repair. 2013, 12, 1037-1042.
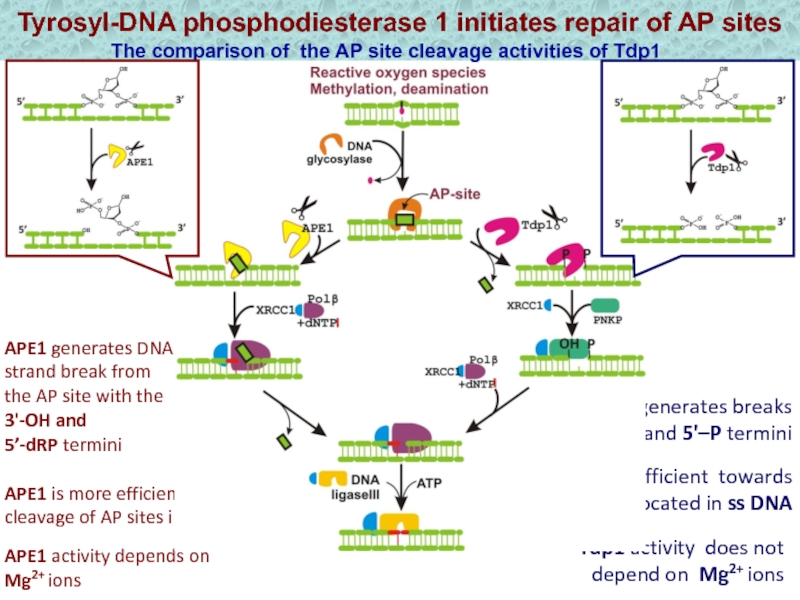
Слайд 18Tyrosyl-DNA phosphodiesterase 1 initiates repair of AP sites
The comparison of
the AP site cleavage activities of Tdp1 and APE1
Tdp1 is
more efficient towards AP sites located in ss DNA Tdp1 generates breaks
with the 3'–P and 5'–P termini
APE1 generates DNA strand break from the AP site with the 3'-OH and
5’-dRP termini
APE1 activity depends on Mg2+ ions
APE1 is more efficient in the cleavage of AP sites in ds DNA
Tdp1 activity does not depend on Mg2+ ions
Слайд 19
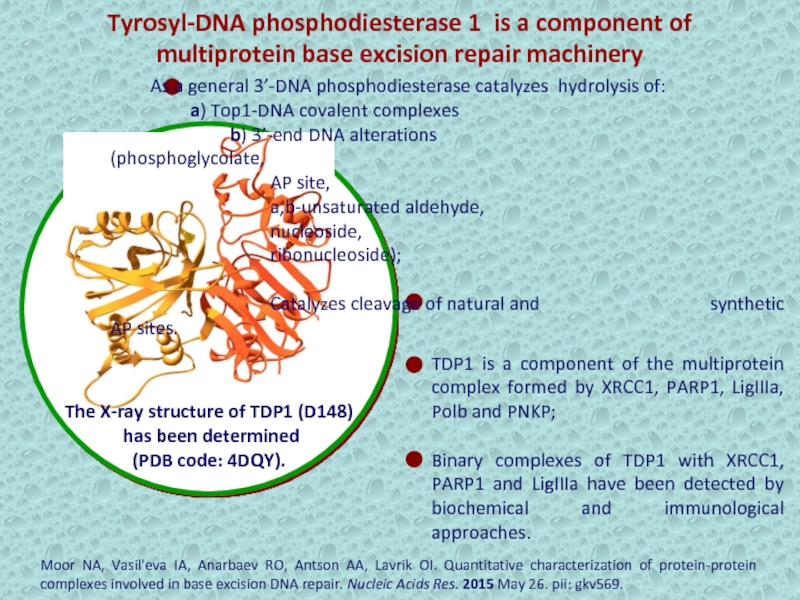
Tyrosyl-DNA phosphodiesterase 1 is a component of multiprotein base excision
repair machinery
TDP1 is a component of the multiprotein complex formed
by XRCC1, PARP1, LigIIIa, Polb and PNKP;Binary complexes of TDP1 with XRCC1, PARP1 and LigIIIa have been detected by biochemical and immunological approaches.
Moor NA, Vasil'eva IA, Anarbaev RO, Antson AA, Lavrik OI. Quantitative characterization of protein-protein complexes involved in base excision DNA repair. Nucleic Acids Res. 2015 May 26. pii: gkv569.
The X-ray structure of TDP1 (D148)
has been determined
(PDB code: 4DQY).
As a general 3’-DNA phosphodiesterase catalyzes hydrolysis of:
a) Top1-DNA covalent complexes
b) 3’-end DNA alterations (phosphoglycolate,
AP site,
a,b-unsaturated aldehyde,
nucleoside,
ribonucleoside);
Catalyzes cleavage of natural and synthetic AP sites.
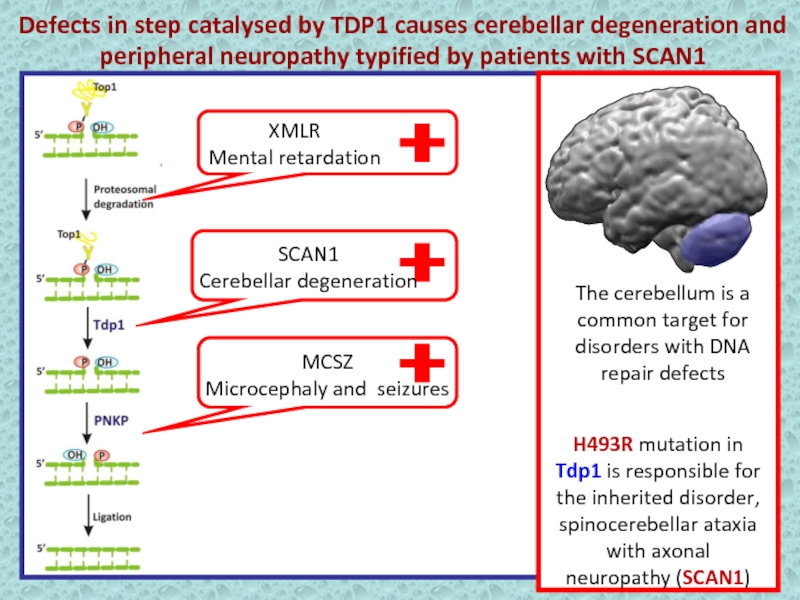
Слайд 20Defects in step catalysed by TDP1 causes cerebellar degeneration and
peripheral neuropathy typified by patients with SCAN1
The cerebellum is
a common target for disorders with DNA repair defectsH493R mutation in Tdp1 is responsible for the inherited disorder, spinocerebellar ataxia with axonal neuropathy (SCAN1)
XMLR
Mental retardation
SCAN1
Cerebellar degeneration
MCSZ
Microcephaly and seizures
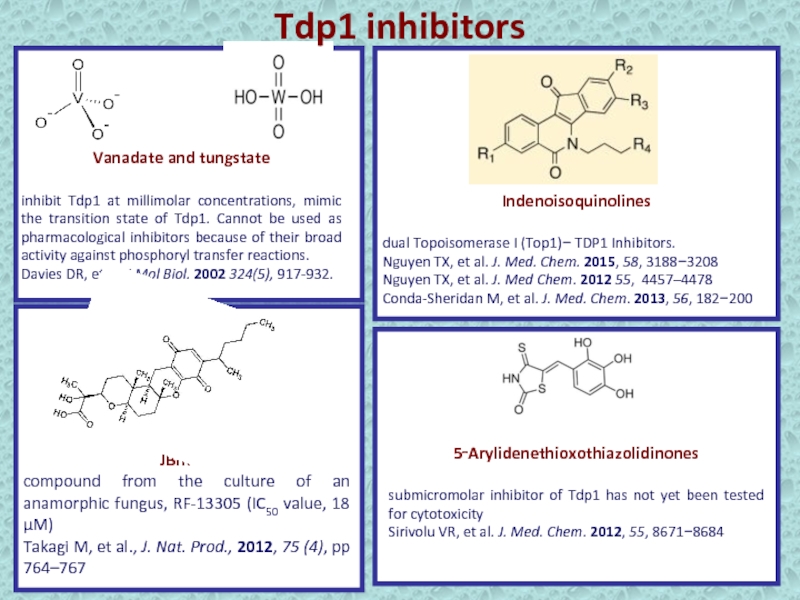
Слайд 22Vanadate and tungstate
inhibit Tdp1 at millimolar concentrations, mimic the transition
state of Tdp1. Cannot be used as pharmacological inhibitors because
of their broad activity against phosphoryl transfer reactions.Davies DR, et al. J Mol Biol. 2002 324(5), 917-932.
Indenoisoquinolines
dual Topoisomerase I (Top1)− TDP1 Inhibitors.
Nguyen TX, et al. J. Med. Chem. 2015, 58, 3188−3208
Nguyen TX, et al. J. Med Chem. 2012 55, 4457–4478
Conda-Sheridan M, et al. J. Med. Chem. 2013, 56, 182−200
5‑Arylidenethioxothiazolidinones
submicromolar inhibitor of Tdp1 has not yet been tested for cytotoxicity
Sirivolu VR, et al. J. Med. Chem. 2012, 55, 8671−8684
JBIR-21,
compound from the culture of an anamorphic fungus, RF-13305 (IC50 value, 18 μM)
Takagi M, et al., J. Nat. Prod., 2012, 75 (4), pp 764–767
Tdp1 inhibitors
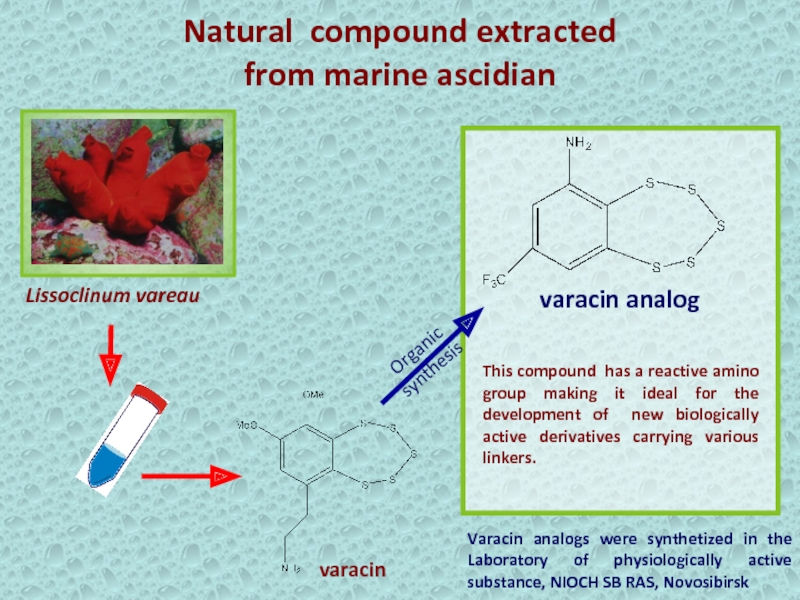
Слайд 23Natural compound extracted
from marine ascidian
Varacin analogs were synthetized
in the Laboratory of physiologically active substance, NIOCH SB RAS,
NovosibirskLissoclinum vareau
varacin
This compound has a reactive amino group making it ideal for the development of new biologically active derivatives carrying various linkers.
varacin analog
Organic
synthesis
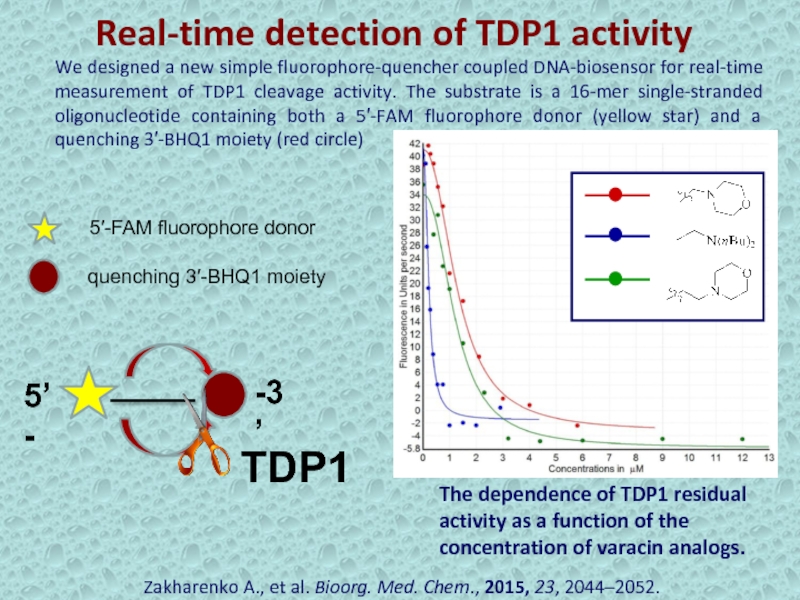
Слайд 24Real-time detection of TDP1 activity
Zakharenko A., et al. Bioorg. Med.
Chem., 2015, 23, 2044–2052.
5’-
-3’
We designed a new simple fluorophore-quencher coupled
DNA-biosensor for real-time measurement of TDP1 cleavage activity. The substrate is a 16-mer single-stranded oligonucleotide containing both a 5′-FAM fluorophore donor (yellow star) and a quenching 3′-BHQ1 moiety (red circle) The dependence of TDP1 residual activity as a function of the concentration of varacin analogs.
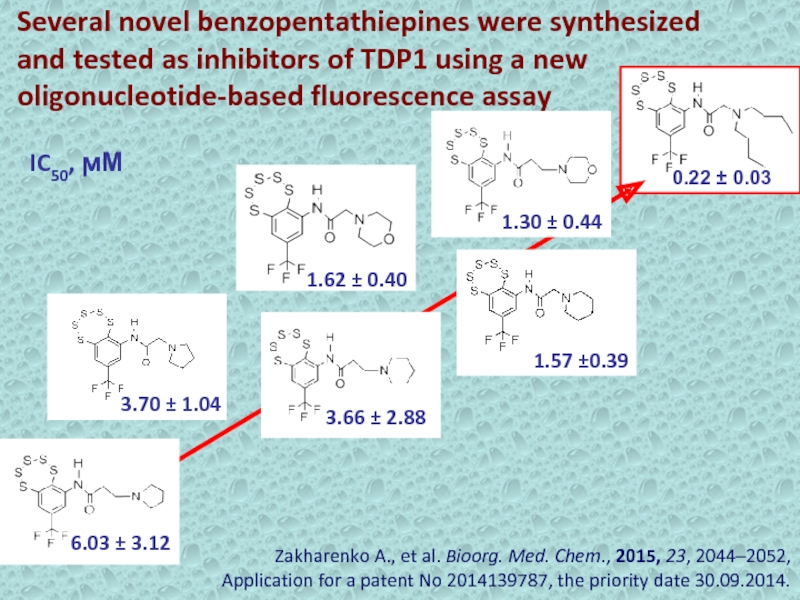
Слайд 25Several novel benzopentathiepines were synthesized and tested as inhibitors of
TDP1 using a new oligonucleotide-based fluorescence assay
0.22 ± 0.03
1.30
± 0.44 1.57 ±0.39
1.62 ± 0.40
3.70 ± 1.04
3.66 ± 2.88
6.03 ± 3.12
IC50, ϻМ
Zakharenko A., et al. Bioorg. Med. Chem., 2015, 23, 2044–2052,
Application for a patent No 2014139787, the priority date 30.09.2014.
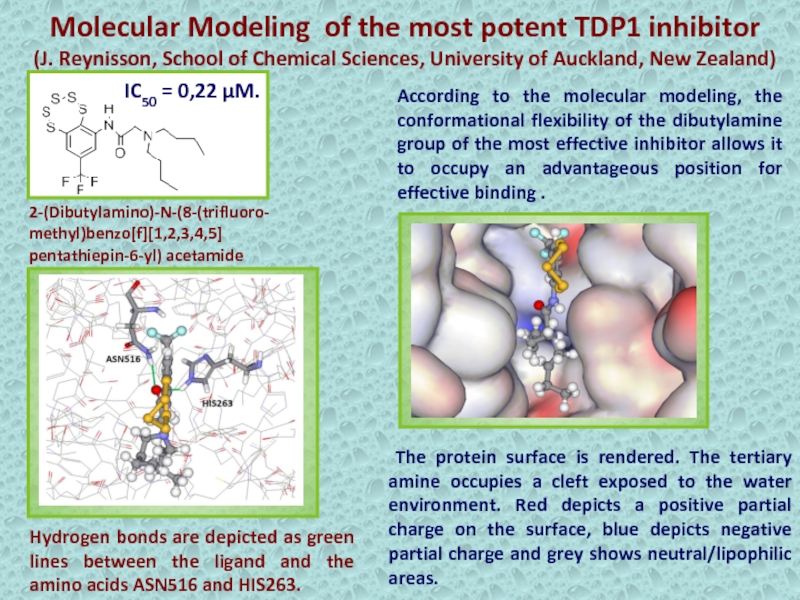
Слайд 26Molecular Modeling of the most potent TDP1 inhibitor
(J. Reynisson, School
of Chemical Sciences, University of Auckland, New Zealand)
According to the
molecular modeling, the conformational flexibility of the dibutylamine group of the most effective inhibitor allows it to occupy an advantageous position for effective binding . The protein surface is rendered. The tertiary amine occupies a cleft exposed to the water environment. Red depicts a positive partial charge on the surface, blue depicts negative partial charge and grey shows neutral/lipophilic areas.
Hydrogen bonds are depicted as green lines between the ligand and the amino acids ASN516 and HIS263.
2-(Dibutylamino)-N-(8-(trifluoro-
methyl)benzo[f][1,2,3,4,5]
pentathiepin-6-yl) acetamide
IC50 = 0,22 µM.
Слайд 27- The cytotoxicity study of the compounds showed that they
caused apoptotic cell death in human mammary adenocarcinoma cell line
MCF-7 and human liver cell carcinoma Hep G2.- Several apoptotic features were observed with light microscopy: condensation of nuclei, shrinkage of the cytoplasm, convolution of outlines and formation of apoptotic bodies.
- The most potent TDP1 inhibitor (dibutylamine derivative) with 200 μM concentration caused 10.5% of the cells to enter apoptosis
Cytotoxicity of varacin analogs
Слайд 28The X-ray structure of TDP1 (D148)
Conclusions
- Tyrosyl-DNA phosphodiestherase 1
is the beneficial target to design the new classes of
anticancer drugs. These drugs are extremely important to improve anticancer therapy by topoisomerase inhibitors.- A new class of TDP1 benzopentathiepine inhibitors active in the low micromolar or high nanomolar range. The derivative containing dibutylamine substituent demonstrates the highest activity. Its potency can be attributed to its high lipophility and a greater conformational flexibility allowing it to effectively fit to the TDP1 active center.
- Tdp1 inhibitors caused apoptotic cell death in cancer cell lines
- The most potent TDP1 inhibitor has the greatest potential to be developed further as an anticancer drug in combination with the established Top1 inhibitors.
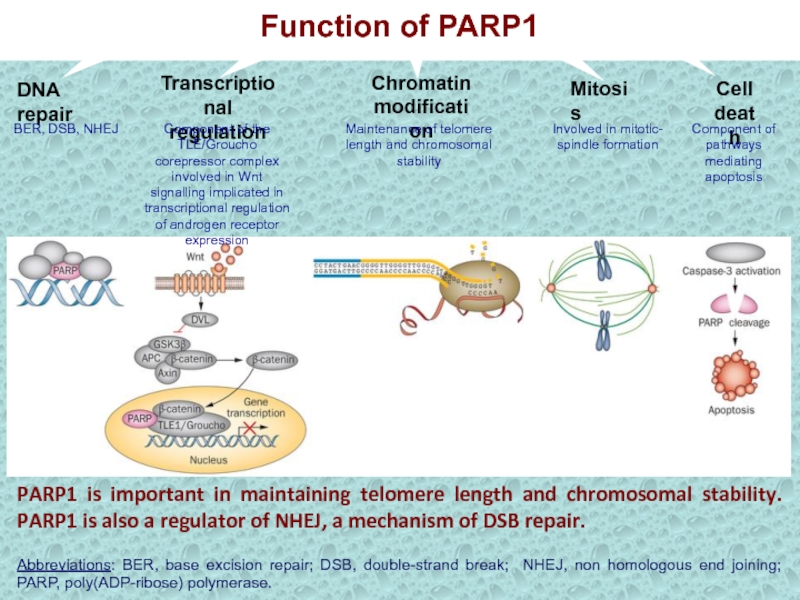
Слайд 29PARP1 is important in maintaining telomere length and chromosomal stability.
PARP1 is also a regulator of NHEJ, a mechanism of
DSB repair.Abbreviations: BER, base excision repair; DSB, double-strand break; NHEJ, non homologous end joining; PARP, poly(ADP-ribose) polymerase.
DNA repair
BER, DSB, NHEJ
Transcriptional
regulation
Component of the TLE/Groucho corepressor complex involved in Wnt signalling implicated in transcriptional regulation of androgen receptor expression
Chromatin
modification
Mitosis
Cell
death
Maintenance of telomere length and chromosomal stability
Involved in mitotic- spindle formation
Component of pathways mediating apoptosis
Function of PARP1
Слайд 30FEN1
APE1
PALF
OGG1
Lig3
APTX
PCNA
MUTYH
TDG
BRAC1
BRAC2
XRCC2
XRCC3
XRCC4
XRCC5
XRCC6
XRCC7
RAD51
MRE-11A
TP53
ERCC1
ERCC2
ERCC5
NBS1
WRN
PARG
Poli
DNTT
TOPO1
PARP1 interacts with the proteins taking part in DNA
repair
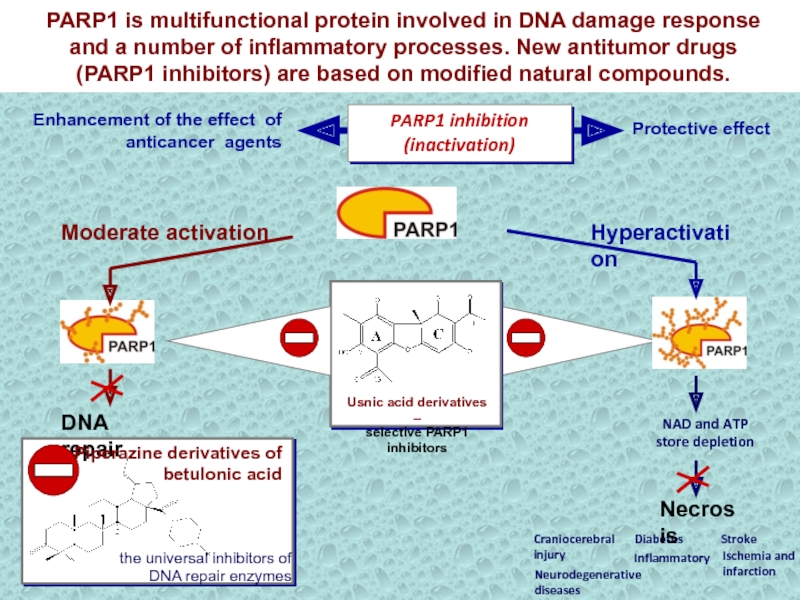
Слайд 31NAD and ATP store depletion
Neurodegenerative
diseases
Stroke
Craniocerebral
injury
Ischemia and
infarction
Diabetes
Inflammatory
Usnic
acid derivatives –
selective PARP1 inhibitors
Enhancement of the effect of
anticancer agentsProtective effect
PARP1 is multifunctional protein involved in DNA damage response and a number of inflammatory processes. New antitumor drugs (PARP1 inhibitors) are based on modified natural compounds.
PARP1 inhibition (inactivation)
Hyperactivation
Moderate activation
DNA repair
Piperazine derivatives of betulonic acid
the universal inhibitors of DNA repair enzymes
Necrosis
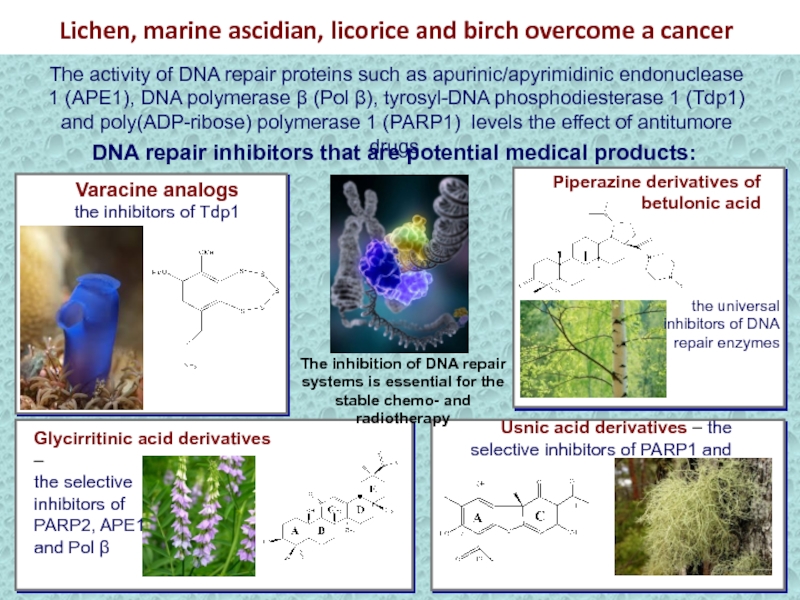
Слайд 32Lichen, marine ascidian, licorice and birch overcome a cancer
Piperazine derivatives
of betulonic acid
Varacine analogs
the inhibitors of Tdp1
Glycirritinic acid derivatives –
the selective
inhibitors of
PARP2, APE1
and Pol β
Usnic acid derivatives – the selective inhibitors of PARP1 and Tdp1
DNA repair inhibitors that are potential medical products:
The inhibition of DNA repair systems is essential for the stable chemo- and radiotherapy
The activity of DNA repair proteins such as apurinic/apyrimidinic endonuclease 1 (АРЕ1), DNA polymerase β (Pol β), tyrosyl-DNA phosphodiesterase 1 (Tdp1) and poly(ADP-ribose) polymerase 1 (PARP1) levels the effect of antitumore drugs.
the universal inhibitors of DNA repair enzymes
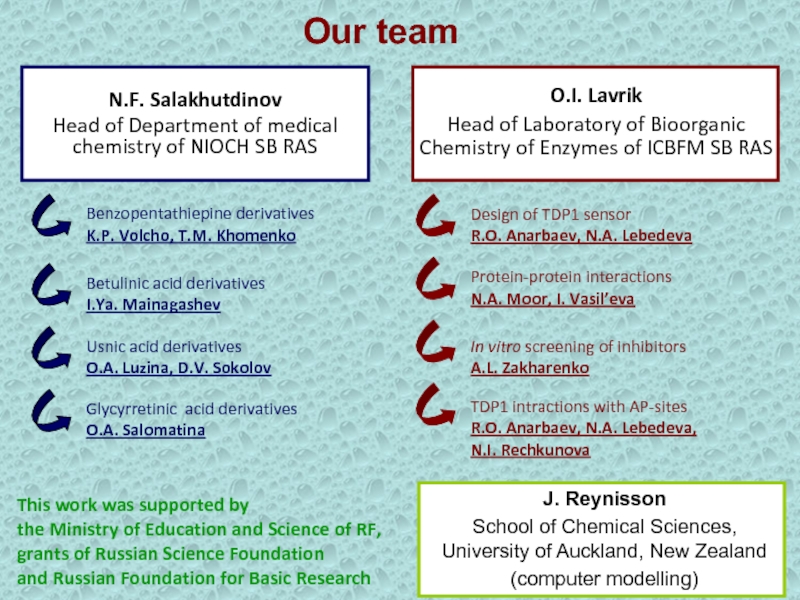
Слайд 33Our team
N.F. Salakhutdinov
Head of Department of medical chemistry of NIOCH
SB RAS
Benzopentathiepine derivatives
K.P. Volcho, T.M. Khomenko
Usnic acid derivatives
O.A. Luzina, D.V.
SokolovBetulinic acid derivatives
I.Ya. Mainagashev
Glycyrretinic acid derivatives
O.A. Salomatina
O.I. Lavrik
Head of Laboratory of Bioorganic Chemistry of Enzymes of ICBFM SB RAS
Design of TDP1 sensor
R.O. Anarbaev, N.A. Lebedeva
In vitro screening of inhibitors
A.L. Zakharenko
TDP1 intractions with AP-sites
R.O. Anarbaev, N.A. Lebedeva, N.I. Rechkunova
J. Reynisson
School of Chemical Sciences, University of Auckland, New Zealand
(computer modelling)
Protein-protein interactions
N.A. Moor, I. Vasil’eva
This work was supported by
the Ministry of Education and Science of RF, grants of Russian Science Foundation
and Russian Foundation for Basic Research
Слайд 36Top1 inhibitors
Camptothecin
and its clinical derivatives
Topotecan
irinotecan
Belotecan
Topovale
Arc-111
Indenoisoquinolines
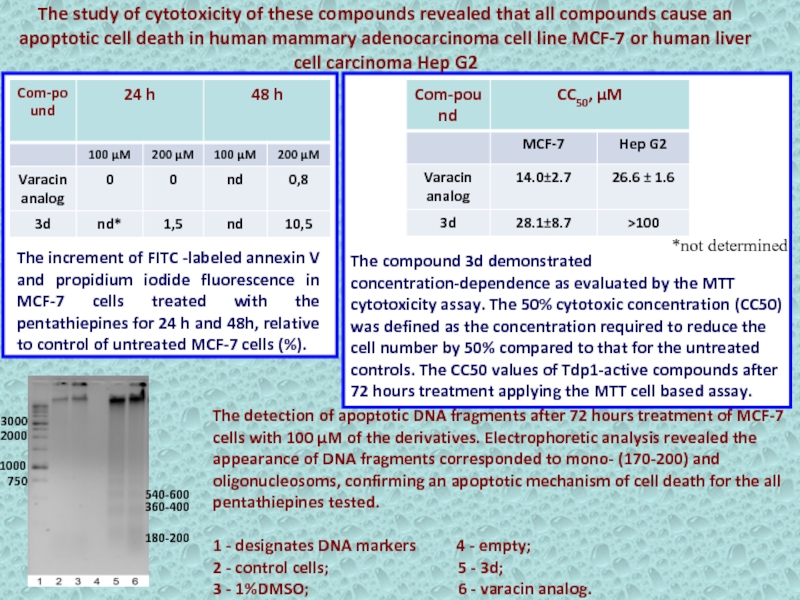
Слайд 37The increment of FITC -labeled annexin V and propidium iodide
fluorescence in MCF-7 cells treated with the pentathiepines for 24
h and 48h, relative to control of untreated MCF-7 cells (%).*not determined
The study of cytotoxicity of these compounds revealed that all compounds cause an apoptotic cell death in human mammary adenocarcinoma cell line MCF-7 or human liver cell carcinoma Hep G2
The compound 3d demonstrated concentration-dependence as evaluated by the MTT cytotoxicity assay. The 50% cytotoxic concentration (CC50) was defined as the concentration required to reduce the cell number by 50% compared to that for the untreated controls. The CC50 values of Tdp1-active compounds after 72 hours treatment applying the MTT cell based assay.
The detection of apoptotic DNA fragments after 72 hours treatment of MCF-7 cells with 100 μM of the derivatives. Electrophoretic analysis revealed the appearance of DNA fragments corresponded to mono- (170-200) and oligonucleosoms, confirming an apoptotic mechanism of cell death for the all pentathiepines tested.
1 - designates DNA markers 4 - empty;
2 - control cells; 5 - 3d;
3 - 1%DMSO; 6 - varacin analog.
750
1000
2000
3000
180-200
360-400
540-600
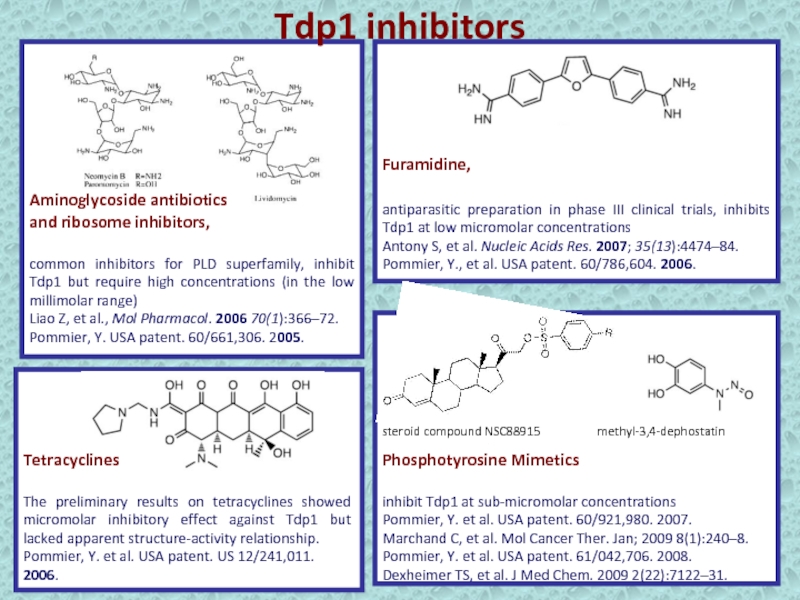
Слайд 38Tdp1 inhibitors
Aminoglycoside antibiotics
and ribosome inhibitors,
common inhibitors for PLD
superfamily, inhibit Tdp1 but require high concentrations (in the low
millimolar range)Liao Z, et al., Mol Pharmacol. 2006 70(1):366–72.
Pommier, Y. USA patent. 60/661,306. 2005.
Furamidine,
antiparasitic preparation in phase III clinical trials, inhibits Tdp1 at low micromolar concentrations
Antony S, et al. Nucleic Acids Res. 2007; 35(13):4474–84.
Pommier, Y., et al. USA patent. 60/786,604. 2006.
Tetracyclines
The preliminary results on tetracyclines showed micromolar inhibitory effect against Tdp1 but lacked apparent structure-activity relationship.
Pommier, Y. et al. USA patent. US 12/241,011. 2006.
Phosphotyrosine Mimetics
inhibit Tdp1 at sub-micromolar concentrations
Pommier, Y. et al. USA patent. 60/921,980. 2007.
Marchand C, et al. Mol Cancer Ther. Jan; 2009 8(1):240–8.
Pommier, Y. et al. USA patent. 61/042,706. 2008.
Dexheimer TS, et al. J Med Chem. 2009 2(22):7122–31.
methyl-3,4-dephostatin
steroid compound NSC88915





![Human Tyrosyl-DNA Phosphodiesterase 1 : new
activities and development of DNA lesionsEndogenousExogenousabasic sites8-oxoguanosine5-hydroxycytosinecytosine methylationphoto dimersO6-methylguanineN6-ethenoadenineN2-dG-benzo[a]pyreneadduct DNA lesionsEndogenousExogenousabasic sites8-oxoguanosine5-hydroxycytosinecytosine methylationphoto dimersO6-methylguanineN6-ethenoadenineN2-dG-benzo[a]pyreneadduct](/img/thumbs/84bffbba7c47eda187c79d7817e32e7d-800x.jpg)