Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Hypersaline environments High UV radiation Desiccation Lecture 13-14 Life under
Содержание
- 1. Hypersaline environments High UV radiation Desiccation Lecture 13-14 Life under
- 2. https://microbiologysociety.org/publication/past-issues/real-superheroes/
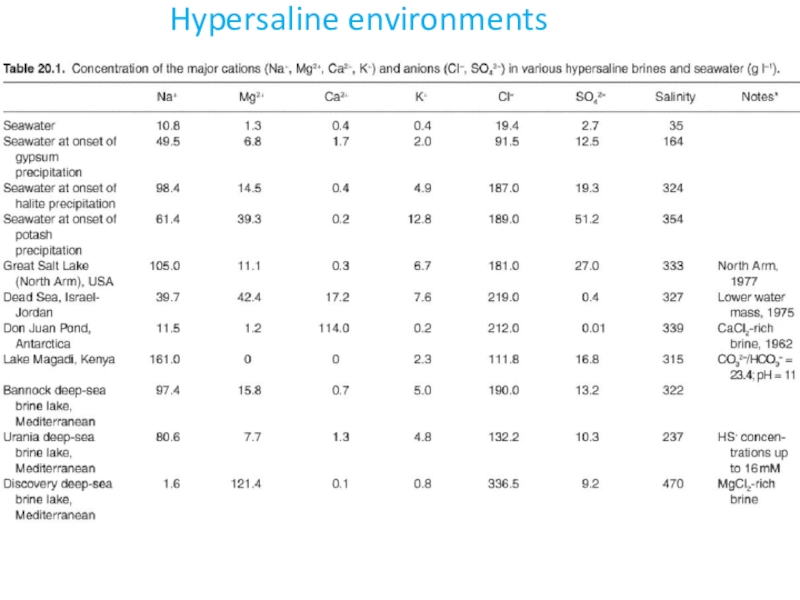
- 3. Hypersaline environments
- 4. Hypersaline environments
- 5. Mechanisms of adaption to high salinityAn organism
- 6. Protein adaptations to extremely high salinityHalophilic organisms
- 7. Protein adaptations to extremely high salinityThese features
- 8. Protein adaptations to extremely high salinityBoth salt
- 9. Protein adaptations to extremely high salinityStudies of
- 10. Extreme for halophilesThe most convincing demonstration and
- 11. Halobacterium salinarumHalobacterium salinarum is an extremophile superhero
- 12. Halobacterium salinarumThe red colour of extremely salty
- 13. H. salinarum adaptations to salinityFor H. salinarum
- 14. Interlude 1: Dunaliella salinaThe main photosynthetic microbe
- 15. Attempts have been made to exploit the
- 16. Interlude 2: Haloquadratum walsbyiH. salinarum has a
- 17. Living inside salt crystalsAlthough a crystal of
- 18. Living inside salt crystalsBut consider: a brine
- 19. Adaptations to high levels of radiationHypersaline environments
- 20. Retinal proteins of H. salinarumH. salinarum contains
- 21. HalorhodopsinBacteriorhodopsin acts as a proton pumpCaptures light
- 22. Microbial rhodopsinsInoue et al., 2014
- 23. ChannelrhodopsinsChannelrhodopsins (ChR) function as light-gated ion channelsThey
- 24. OptogeneticsExpressed in cells of other organisms, ChRs
- 25. OptogeneticsHalorhodopsin NpHR (Cl- pump) quickly and reversibly
- 26. Wietek et al., 2015 https://www.nature.com/articles/srep14807Further engineering of channelrhodopsins
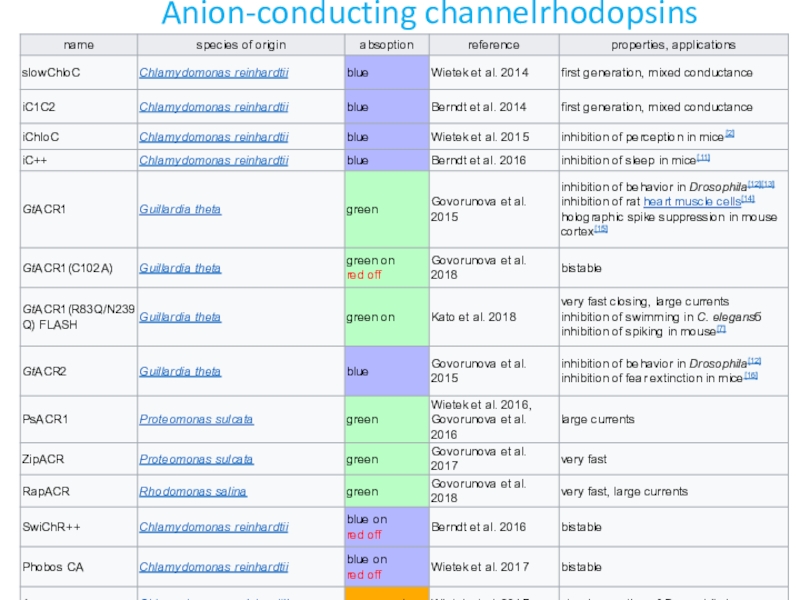
- 27. Anion-conducting channelrhodopsins
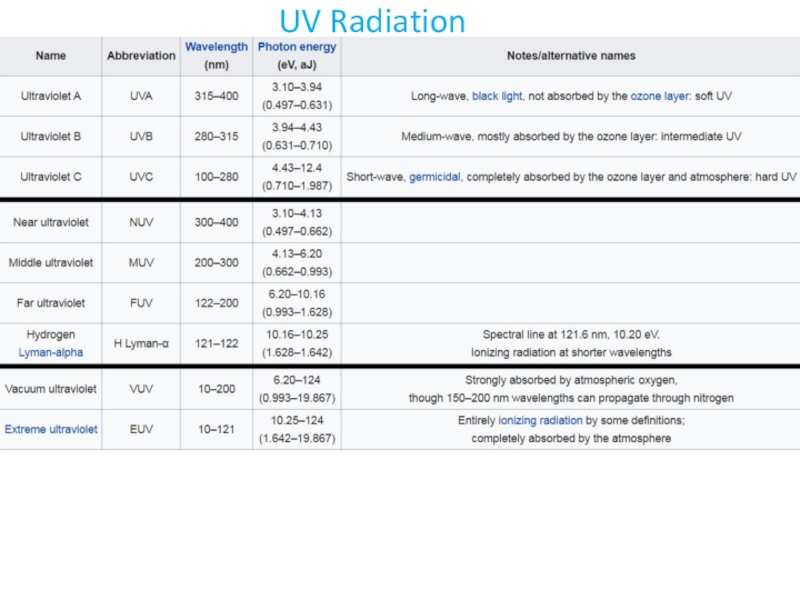
- 28. UV Radiation
- 29. Biological Effects of UV RadiationExposure to UV-B
- 30. Tolerance mechanismsThe sensitivity of aquatic organisms to
- 31. AvoidanceExternal features such as mucilage, membranes, the
- 32. Sunscreens: Mycosporine-like amino acids Aquatic organisms protect
- 33. Screening and quenchinga carotenoidmelanin
- 34. Repair of UV-B-induced DNA damageThere are large
- 35. Repair of UV-B-induced DNA damage“Dark repair” uses
- 36. Deinococcus radiodurans Radioresistant thermophilic bacterium Deinococcus radiodurans
- 37. Deinococcus radiodurans: DNA repair D. radiodurans can
- 38. Deinococcus radiodurans: antioxidantsD. radiodurans has remarkably many
- 39. Deinococcus radiodurans: Applications D. radiodurans has been
- 40. Part 2. Tardigrades - Life without water
- 41. Tardigrades: a Metazoan polyextremophileMilnesium tardigradumSurvive the temperatures:
- 42. Tardigrades: a Metazoan polyextremophile3 tardigrade species have
- 43. Anhydrobiosis in tardigradesTwo main strategies to cope
- 44. Anhydrobiosis in tardigradesAfter tun formation, the cuticle
- 45. Anhydrobiosis in tardigradesThe level of trehalose increases
- 46. Molecular responses to radiationA tardigrade-unique protein, Damage
- 47. Longevity vs DNA damageWhat are the consequences
- 48. Longevity vs DNA damageThere is evidence that
- 49. Longevity vs DNA damageSurvival during anhydrobiosis could
- 50. Tardigrades in biomedical researchHow genome integrity is
- 51. Tardigrades in biomedical researchOxidative stress is a
- 52. Скачать презентанцию
Слайды и текст этой презентации
Слайд 1Hypersaline environments
High UV radiation
Desiccation
Lecture 13-14
Life under extreme conditions:
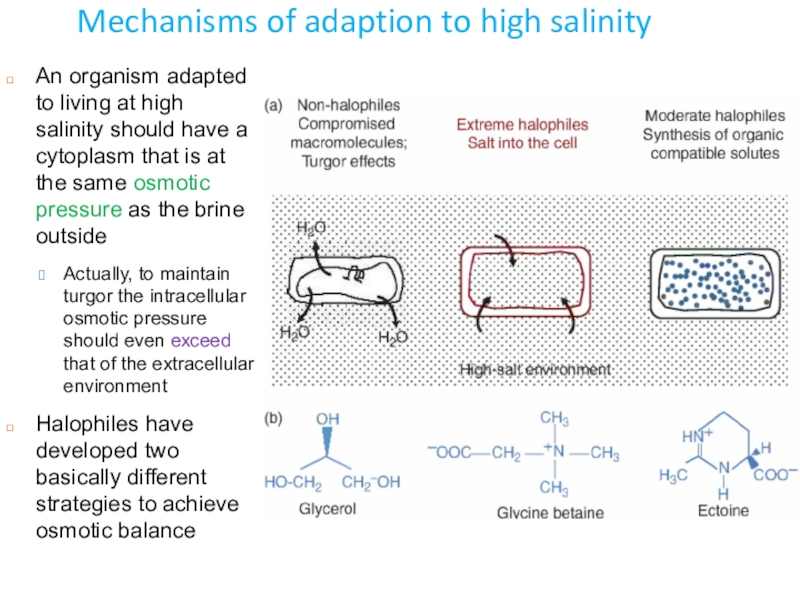
Слайд 5Mechanisms of adaption to high salinity
An organism adapted to living
at high salinity should have a cytoplasm that is at
the same osmotic pressure as the brine outsideActually, to maintain turgor the intracellular osmotic pressure should even exceed that of the extracellular environment
Halophiles have developed two basically different strategies to achieve osmotic balance
Слайд 6Protein adaptations to extremely high salinity
Halophilic organisms thrive under extremely
ionic conditions caused by high concentrations of metal ions
Evolved
various strategies to cope with stress caused by this extreme environmentThey can accumulate in the cytoplasm both inorganic salts and small organic molecules like amino acids, sugars or polyols
Until the intracellular osmolarity equals the extracellular one, preventing osmotic shock
This leaves the organism in a quandary:
while they are in no danger of osmotic shock
they now must have proteins, nucleic acids and other cellular molecules able to function under extremely ionic conditions
Halophilic proteins rely on salt for proper folding
To accomplish this, they have some common adaptations
An increased number of acidic residues on the protein’s surface
Decreased amount of hydrophobic residues
And multiple amino acid insertions (peptide insertions) in the protein that could increase enzyme flexibility
Слайд 7Protein adaptations to extremely high salinity
These features make halophiles and
their proteins interesting study subjects
Most work in this area indicate
that halophilic proteins sustain their structure and physiological activities at high salt concentrationbut not necessarily at low concentrations
Many enzymes function properly only in the presence of high Na+ and/or K+
suggests that their structures are properly folded only under these ionic conditions
A gradual decrease of salt concentration leads to the unfolding of protein
meaning that proteins cannot function outside a particular salt concentration range
A salient feature of halophilic proteins is enrichment with acidic amino acids
which reside on the protein’s surface when folded
In this way their carboxylic acid side chain compete with cations for water molecules
and/or could bind metal cations
which would prevent aggregation through electrostatic repulsion and/or hydration of the protein
This adaptation alone probably accounts for most of the halophilic proteins’ stability
They also have an optimum pH in the neutral range, which makes acidic amino acids charged as this is above their pI
Слайд 8Protein adaptations to extremely high salinity
Both salt concentration and pH
contribute to electrostatic stability of the proteins
There are fewer hydrophobic
interactions in the core of halophilic proteins The hydrophobic effect is stronger in high salt concentrations
The effect of high salt on flexibility must be balanced by a smaller hydrophobic core
When hydrophobic forces weakened, the flexibility of proteins in high salt concentration increases
In salt concentrations as low as 0.1 M, water becomes less available
Ionic interactions replace water-protein interactions and the high salt concentration inhibits interactions between proteins
Another commonly seen adaptation is the increased number of amino acid and peptide insertions
ranging in size from 2–3 to as many as 30 amino acids
The inserts are enriched in both acidic amino acids and in glycine and proline
Probably also serve to increase the flexibility of the proteins under halophilic conditions
Evidence shows the insertions strongly improve the catalytic activity of halophilic enzymes
Слайд 9Protein adaptations to extremely high salinity
Studies of protein-ion interactions in
Halobacterium salinarum showed a strong preference for group 1 over
group 2 cationsWithin group 1 cations (Li+ thru Cs+), K+ was a preferred cation
The protein retains structure from 1 M to 3 M group 1 ions, but does not have a clear structural pattern when Mg2+ or Ca2+ (group 2 ions) were present
Halophilic proteins have reversible folding pathways
Mediated by the presence of a high number of acidic residues and hydrophilicity
The rate of refolding can be slow, taking up to several days
The proteins unfold when surrounding salt levels decrease and when temperature increases
As KCl is titrated in, the enzymes gradually recover and become active
Salt provides stabilizing interactions which increase thermal stability of halophilic proteins share increased surface charge with thermophiles
The average percent of aa categories per analyzed collection of malate dehydrogenase sequences. Each category is a mix of bacterial and archaeal protein sequences. The gray blocks indicate a significant difference
Слайд 10Extreme for halophiles
The most convincing demonstration and quantification of protein
stability is though thermal melting experiments
In general, as salt
and heat increases, the stability and Tm increases in halophilic proteinsIn case of the cysteinyl-tRNA synthase from Halobacterium salinarum,
2 M KCl increased the Tm of the protein by over 10°C
Whereas it decreased the Tm of the E. coli homolog
Halophilicity can also confer pH stability
The asparatic or glutamic acid side chains that confer stability in the presence of salt can act like a buffer
Balance between different protonation states, which would in turn balance electrostatic effects
Shows that both pH and salt concentration influence enzyme activity
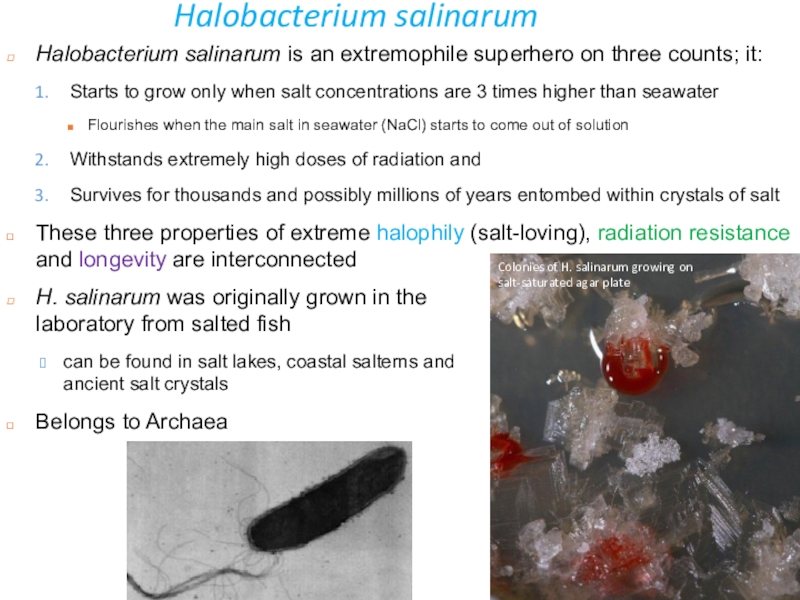
Слайд 11Halobacterium salinarum
Halobacterium salinarum is an extremophile superhero on three counts;
it:
Starts to grow only when salt concentrations are 3 times
higher than seawaterFlourishes when the main salt in seawater (NaCl) starts to come out of solution
Withstands extremely high doses of radiation and
Survives for thousands and possibly millions of years entombed within crystals of salt
These three properties of extreme halophily (salt-loving), radiation resistance and longevity are interconnected
www.flickr.com/photos/juaninda/4249952887
H. salinarum was originally grown in the laboratory from salted fish
can be found in salt lakes, coastal salterns and ancient salt crystals
Belongs to Archaea
Colonies of H. salinarum growing on salt-saturated agar plate
Слайд 12Halobacterium salinarum
The red colour of extremely salty environments, like the
Great Salt Lake in Utah, is due to haloarchaea, and
can be seen from spaceForms red or pink colonies because its cell membrane contains bacterioruberins
www.flickr.com/photos/juaninda/4249952887
a salty pond in the Arabian desert
Слайд 13H. salinarum adaptations to salinity
For H. salinarum to grow in
hypersaline environments, it contains a highly concentrated intracellular salt solution,
mainly consisting of KClSo the osmotic pressure inside and outside the cell is balanced
Consequently, all of its proteins are adapted to work under these conditions
If placed in a freshwater lake or even in the ocean, water would flood into the cell
the cell membrane and proteins would lose their structure and the cells would burst open
K+ is actively pumped into the cell
At extremely high salt concentrations protein precipitation will occur
To prevent the salting out of proteins, H. salinarum encodes mainly acidic proteins
The average isoelectric point of its proteins is 5.03
These highly acidic proteins have very negative surface charges and can remain in solution even at very high salt concentrations
This commitment to an extremely salty existence has its advantages
Experiences lower interspecies competition
So it takes an advantage of the large quantity of unclaimed organic matter
And also new organic matter made by photosynthesising halophiles
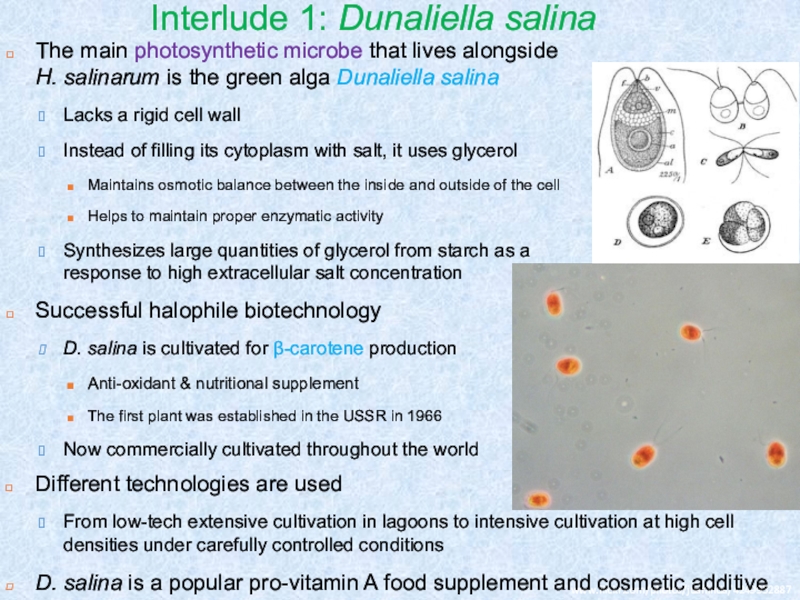
Слайд 14Interlude 1: Dunaliella salina
The main photosynthetic microbe that lives alongside
H. salinarum is the green alga Dunaliella salina
Lacks a
rigid cell wallInstead of filling its cytoplasm with salt, it uses glycerol
Maintains osmotic balance between the inside and outside of the cell
Helps to maintain proper enzymatic activity
Synthesizes large quantities of glycerol from starch as a response to high extracellular salt concentration
Successful halophile biotechnology
D. salina is cultivated for β-carotene production
Anti-oxidant & nutritional supplement
The first plant was established in the USSR in 1966
Now commercially cultivated throughout the world
www.flickr.com/photos/juaninda/4249952887
Different technologies are used
From low-tech extensive cultivation in lagoons to intensive cultivation at high cell densities under carefully controlled conditions
D. salina is a popular pro-vitamin A food supplement and cosmetic additive
Слайд 15Attempts have been made to exploit the high concentrations of
glycerol accumulated by D. salina for its commercial production
Although technically
possible, economic feasibility is low No biotechnological operation exists to exploit the alga for glycerol production
Glycerol leaking from the algal cells provides an excellent source of carbon and energy for H. salinarum
H. salinarum provides nutrients to stimulate growth of the alga in return – a form of symbiosis
www.flickr.com/photos/juaninda/4249952887
Сиваш
Interlude 1: Dunaliella salina
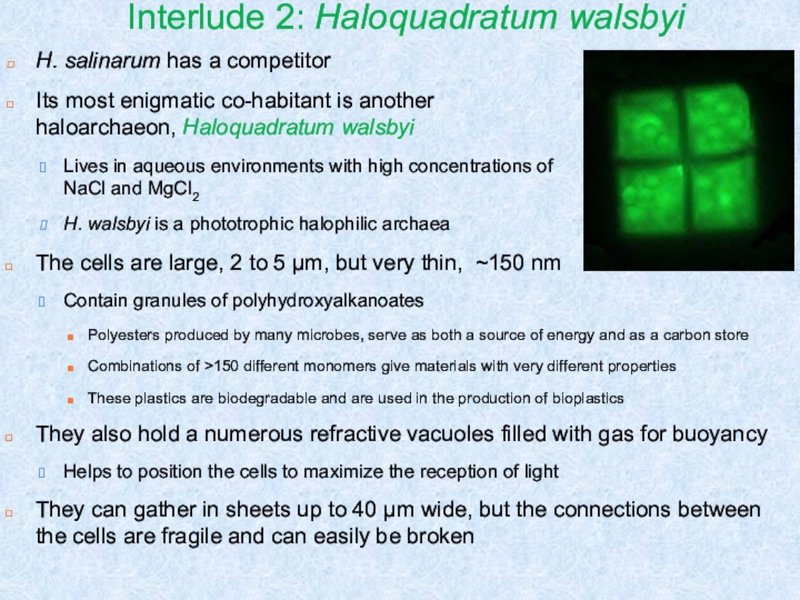
Слайд 16Interlude 2: Haloquadratum walsbyi
H. salinarum has a competitor
Its most enigmatic
co-habitant is another haloarchaeon, Haloquadratum walsbyi
Lives in aqueous environments with
high concentrations of NaCl and MgCl2H. walsbyi is a phototrophic halophilic archaea
The cells are large, 2 to 5 μm, but very thin, ~150 nm
Contain granules of polyhydroxyalkanoates
Polyesters produced by many microbes, serve as both a source of energy and as a carbon store
Combinations of >150 different monomers give materials with very different properties
These plastics are biodegradable and are used in the production of bioplastics
They also hold a numerous refractive vacuoles filled with gas for buoyancy
Helps to position the cells to maximize the reception of light
They can gather in sheets up to 40 μm wide, but the connections between the cells are fragile and can easily be broken
Слайд 17Living inside salt crystals
Although a crystal of common salt may
look completely dry, up to 5% of its volume is
liquid in the form of hundreds of brine inclusionsi.e. small reservoirs of salt-saturated brine surrounded by a solid matrix of NaCl
The haloarchaea become trapped inside salt crystals, living in the brine inclusions
Hypersaline environments are dynamic systems that frequently dry up
The strategy employed by H. salinarum et al. enables them to survive within a small-scale aquatic environment
Until the rains come and dissolve the salt crystals, regenerating the brine lake
But what can H. salinarum eat inside brine inclusions?
The repair of H. salinarum proteins and nucleic acids needs organic matter for energy
Is there enough organic matter in the brine inclusions to live for 1000s of years?
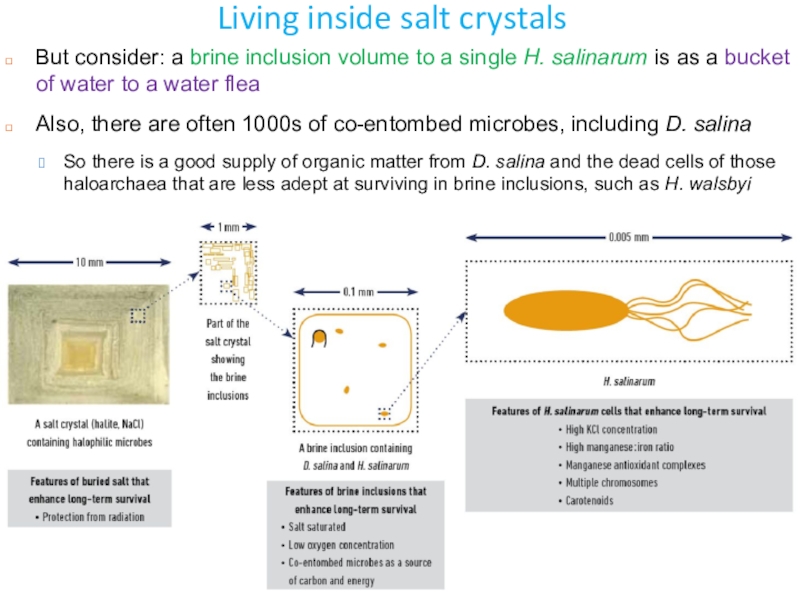
Слайд 18Living inside salt crystals
But consider: a brine inclusion volume to
a single H. salinarum is as a bucket of water
to a water fleaAlso, there are often 1000s of co-entombed microbes, including D. salina
So there is a good supply of organic matter from D. salina and the dead cells of those haloarchaea that are less adept at surviving in brine inclusions, such as H. walsbyi
Слайд 19Adaptations to high levels of radiation
Hypersaline environments are prone to
drying up
Add the high level of UV radiation that is
typical of such environments, get cell damageinduces DNA double-strand breaks
Both desiccation and radiation can damage cells by the production of highly reactive forms of oxygen
So microbes that cope with drying are generally also good at surviving high radiation
H. salinarum has evolved mechanisms that make it one of the most radiation-resistant microbes known
The high cellular concentrations of peptides and the minerals phosphate and Mn, combine to protect cellular proteins
The proteins include enzymes that repair damaged nucleic acids
Also, it has multiple copies of the chromosome and an efficient means of repairing and recombining DNA fragments
All this ensures that genetic material stays intact
The carotenoids and high cellular concentrations of KCl also provide radiation protection
Слайд 20Retinal proteins of H. salinarum
H. salinarum contains 4 retinal proteins
photosynthetic pigments with a retinal chromophore involved in light energy
conversion and signal transduction Bacteriorhodopsin
The pigment that permits H. salinarum to grow with light as only energy source
A light-driven proton pump which converts light energy into a proton gradient
The energy stored in the proton gradient can be used in different ways, e.g. for generation of ATP via ATP synthase
Halorhodopsin
a light-driven Cl- pump that permits H. salinarum to maintain the high internal salt concentration upon growth
Sensory rhodopsin I
involved in phototaxis, mediates the photophilic response to orange and also the photophobic response to UV light forms a complex with the transducer protein htr1
Sensory rhodopsin II
involved in phototaxis, mediates the photophobic response to blue light
forms a complex with the transducer protein htr2
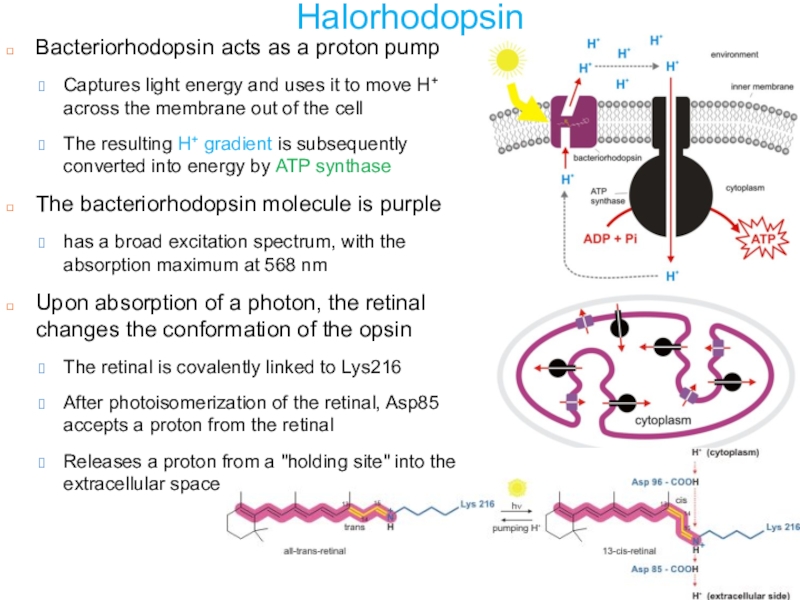
Слайд 21Halorhodopsin
Bacteriorhodopsin acts as a proton pump
Captures light energy and uses
it to move H+ across the membrane out of the
cellThe resulting H+ gradient is subsequently converted into energy by ATP synthase
The bacteriorhodopsin molecule is purple
has a broad excitation spectrum, with the absorption maximum at 568 nm
Upon absorption of a photon, the retinal changes the conformation of the opsin
The retinal is covalently linked to Lys216
After photoisomerization of the retinal, Asp85 accepts a proton from the retinal
Releases a proton from a "holding site" into the extracellular space
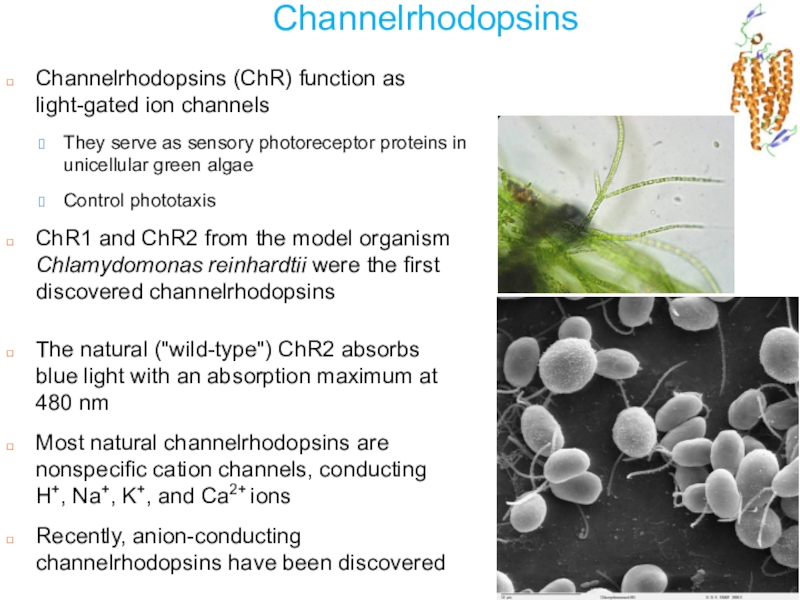
Слайд 23Channelrhodopsins
Channelrhodopsins (ChR) function as light-gated ion channels
They serve as sensory
photoreceptor proteins in unicellular green algae
Control phototaxis
ChR1 and ChR2 from
the model organism Chlamydomonas reinhardtii were the first discovered channelrhodopsinsThe natural ("wild-type") ChR2 absorbs blue light with an absorption maximum at 480 nm
Most natural channelrhodopsins are nonspecific cation channels, conducting H+, Na+, K+, and Ca2+ ions
Recently, anion-conducting channelrhodopsins have been discovered
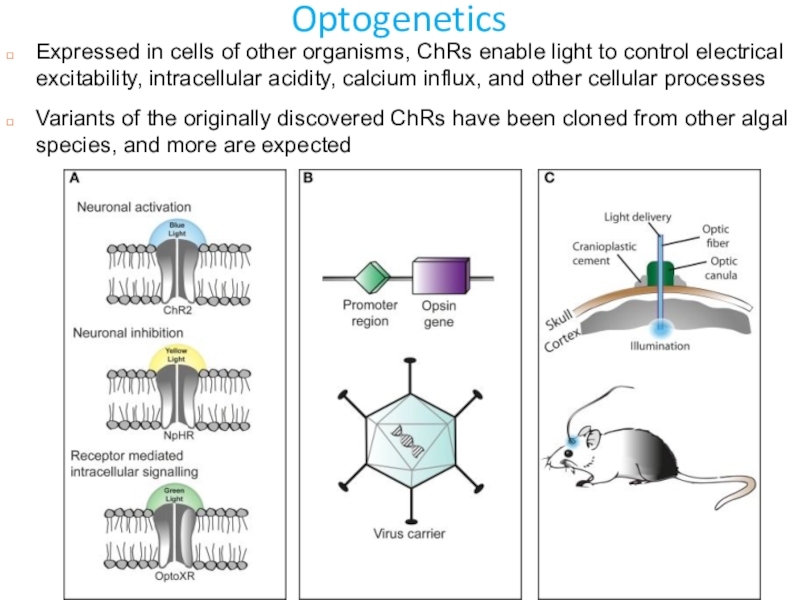
Слайд 24Optogenetics
Expressed in cells of other organisms, ChRs enable light to
control electrical excitability, intracellular acidity, calcium influx, and other cellular
processesVariants of the originally discovered ChRs have been cloned from other algal species, and more are expected
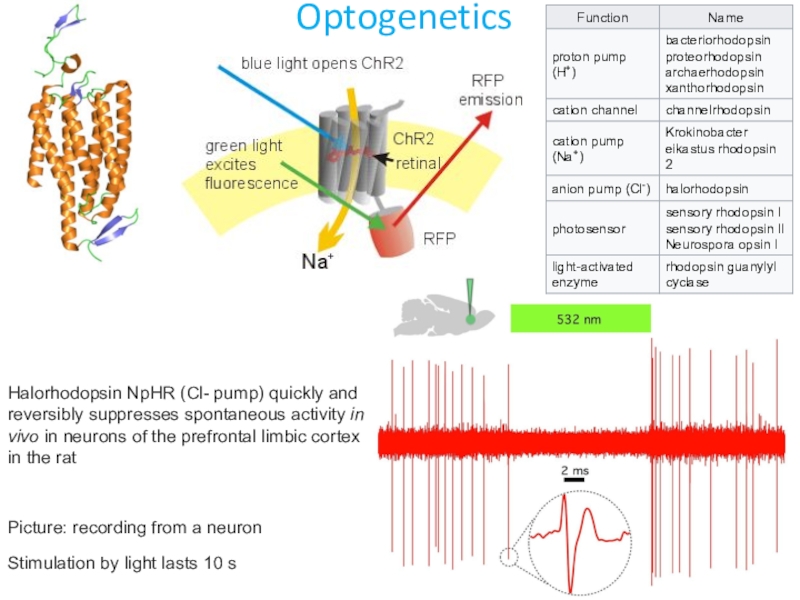
Слайд 25Optogenetics
Halorhodopsin NpHR (Cl- pump) quickly and reversibly suppresses spontaneous activity
in vivo in neurons of the prefrontal limbic cortex in the
ratPicture: recording from a neuron
Stimulation by light lasts 10 s
Слайд 26Wietek et al., 2015 https://www.nature.com/articles/srep14807
Further engineering of channelrhodopsins
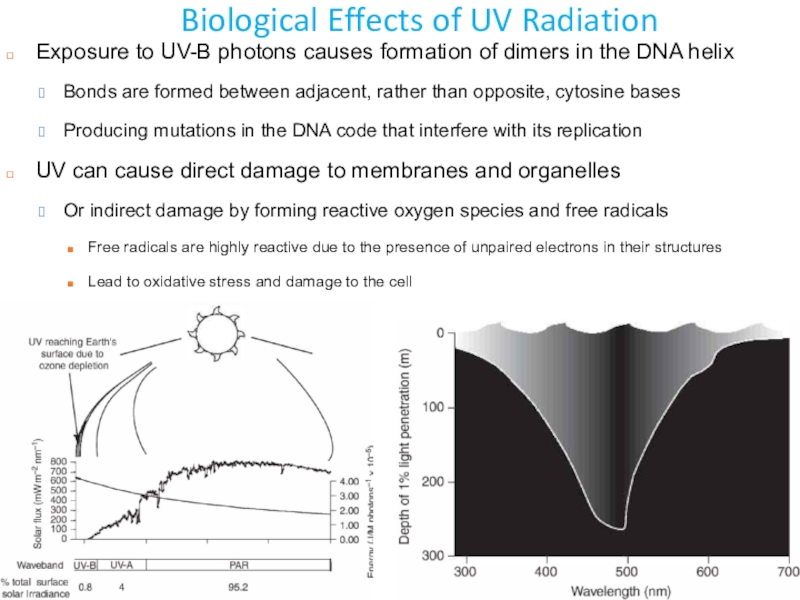
Слайд 29Biological Effects of UV Radiation
Exposure to UV-B photons causes formation
of dimers in the DNA helix
Bonds are formed between adjacent,
rather than opposite, cytosine basesProducing mutations in the DNA code that interfere with its replication
UV can cause direct damage to membranes and organelles
Or indirect damage by forming reactive oxygen species and free radicals
Free radicals are highly reactive due to the presence of unpaired electrons in their structures
Lead to oxidative stress and damage to the cell
Слайд 30Tolerance mechanisms
The sensitivity of aquatic organisms to UV-B damage varies
greatly due to differences in the extent of UV exposure
and damage prevention/repairFour mechanisms can be used to enhance an organism's tolerance to UV-B exposure: avoidance, screening, quenching and repair
The widespread occurrence of these tolerance mechanisms indicates that UV exposure is an important factor in aquatic environments
Avoidance and screening by pigments reduces the incoming UV-B radiation
Avoidance is beyond the capability of plankton organisms that are largely unable to control their depth
Organisms that can control their depth may avoid high UV-B by migrating downward or retreating to shade (negative phototaxis)
However, there are no visual pigments capable of detecting UV-B
many organisms can detect UV-A wavelengths
UV-B can damage an organism without detection
but is it really that important to detect UV-B separately?
Yes, depletion of stratospheric ozone enhances only UV-B wavelengths
Слайд 31Avoidance
External features such as mucilage, membranes, the silica shell of
diatoms (frustules), the theca of dinoflagellates and the scales of
naked flagellates might protect unicellular organisms from UV-B exposureСлайд 32Sunscreens: Mycosporine-like amino acids
Aquatic organisms protect themselves from UV-B
by producing UV-absorbing sunscreens
e.g. mycosporine-like amino acids (MAAs), carotenoids and
melaninMAAs are produced by algae (phytoplankton and macrophytes)
Can be transferred to higher organisms via the food chain
which concentrate and locate the MAAs to protect UV-B sensitive organelles and life stages
>30 different MAAs identified to date
Can transfer energy to opsins to enable indirect detection of UV-B
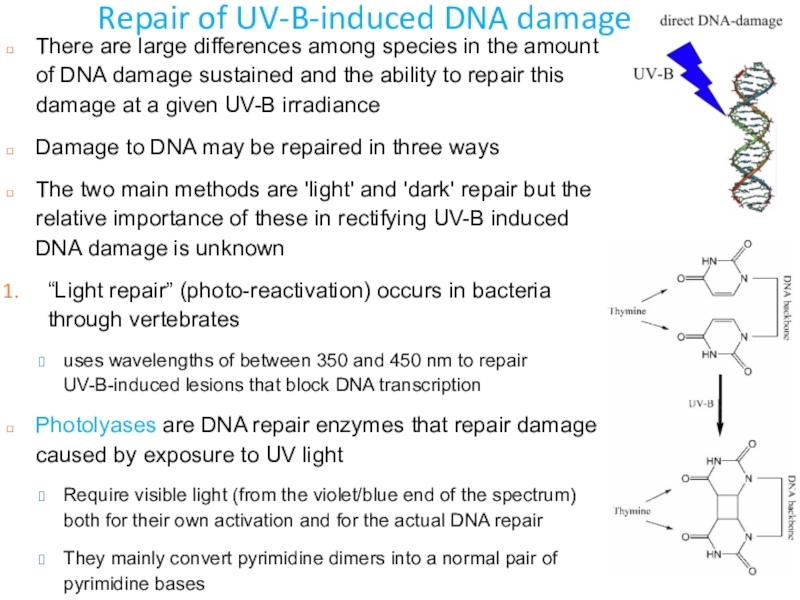
Слайд 34Repair of UV-B-induced DNA damage
There are large differences among species
in the amount of DNA damage sustained and the ability
to repair this damage at a given UV-B irradianceDamage to DNA may be repaired in three ways
The two main methods are 'light' and 'dark' repair but the relative importance of these in rectifying UV-B induced DNA damage is unknown
“Light repair” (photo-reactivation) occurs in bacteria through vertebrates
uses wavelengths of between 350 and 450 nm to repair UV-B-induced lesions that block DNA transcription
Photolyases are DNA repair enzymes that repair damage caused by exposure to UV light
Require visible light (from the violet/blue end of the spectrum) both for their own activation and for the actual DNA repair
They mainly convert pyrimidine dimers into a normal pair of pyrimidine bases
Слайд 35Repair of UV-B-induced DNA damage
“Dark repair” uses enzymes to remove
and replace damaged parts of DNA
Types: proofreading by DNA polymerase;
excision repairs; spontaneous repair by DNA double helix; homologous and non-homologous DNA repair; mismatch repair of single-base mispairsPost-replication repair (“SOS repair”)
via reactive overexpression of heat-shock proteins
Or, in E. coli, the SOS response is a post-replication DNA repair system that allows DNA replication to bypass lesions or errors in the DNA
Differences in the effectiveness of tolerance mechanisms cause large intra- and inter-specific variations in UV-B sensitivity
that are mediated by environmental factors such as temperature, nutrients and salinity
DNA damage can varied 100-fold among species in general and over 10-fold among members of the same genus
Слайд 36Deinococcus radiodurans
Radioresistant thermophilic bacterium Deinococcus radiodurans is used for
the treatment of mixed radioactive wastes containing ionic mercury
Polyextremophile,
the most radiation resistant organism described to dateCan withstand an acute dose of 5,000 grays (Gy), or 500,000 rad
Humans die after exposure of 5 Gy
Can survive cold, dehydration, vacuum, and low pH, “the world's toughest bacterium”
But does not thrive under such conditions
D. radiodurans can uniquely repair both single- and double-stranded DNA
In its stationary phase, each bacterial cell contains 4 copies of genome
when rapidly multiplying, each bacterium contains 8-10 copies of the genome
Four cells normally stick together, forming a tetrad; does not form endospores and is non-motile
An obligate aerobic chemoorganoheterotroph
i.e., uses oxygen to derive energy from organic compounds
Слайд 37Deinococcus radiodurans: DNA repair
D. radiodurans can repair DNA from
many small fragments of a chromosome within 12–24 hours
Can repair
100s, sometimes 1000s, of radiation-induced double-strand breaks (DSBs) per cell Standard species’ cells can repair only a few
The basic mechanism of DSB repair in D. radiodurans is the extended synthesis-dependent strand annealing
This process does not introduce any more mutations than a normal round of replication would
DNA in D. radiodurans is organized into tightly packed toroids, which may facilitate DNA repair
Слайд 38Deinococcus radiodurans: antioxidants
D. radiodurans has remarkably many genes encoding catabolic
enzymes
such as phosphatases, nucleases, and proteases
degradation of proteins produces
amino acids and peptides that can be reused during the energetically demanding recovery periodgive rise to the pool of small antioxidant molecules
Amino acids serve as ROS scavengers
There are at least three independent systems to maintain low ROS levels
Increased ROS-scavenging and ROS-detoxifying activities
High levels of constitutive catalase and superoxide dismutase activity, increased amount of manganese complexes, and small antioxidant molecules
Altered metabolic activities that result in a decreased ROS production
e.g. glyoxylate bypass of the TCA cycle
Reduction of the proteins containing Fe-S clusters, as well as reduction of the number of respiratory chain enzymes
Divalent iron is a source of production of the most reactive ROS, the hydroxyl radical 'OH-
Слайд 39Deinococcus radiodurans: Applications
D. radiodurans has been genetically modified for
bioremediation applications
Bioremediation aims to return a contaminated environment to its
natural conditionIn case of nuclear contamination, ionizing radiation limits the amount of potentially useful microorganisms
D. radiodurans was engineered to consume and digest solvents and heavy metals in radioactive environments
The mercuric reductase gene has been cloned from E. coli into Deinococcus to detoxify the ionic mercury residue
frequently found in radioactive waste generated from nuclear weapons manufacture
Another strain of D. radiodurans can detoxify both mercury and toluene in mixed radioactive wastes
D. radiodurans strains with genes encoding a non-specific acid phosphatase from Salmonella enterica or the alkaline phosphatase from Sphingomonas are tested for bioprecipitation of uranium in acid and alkaline solutions, respectively
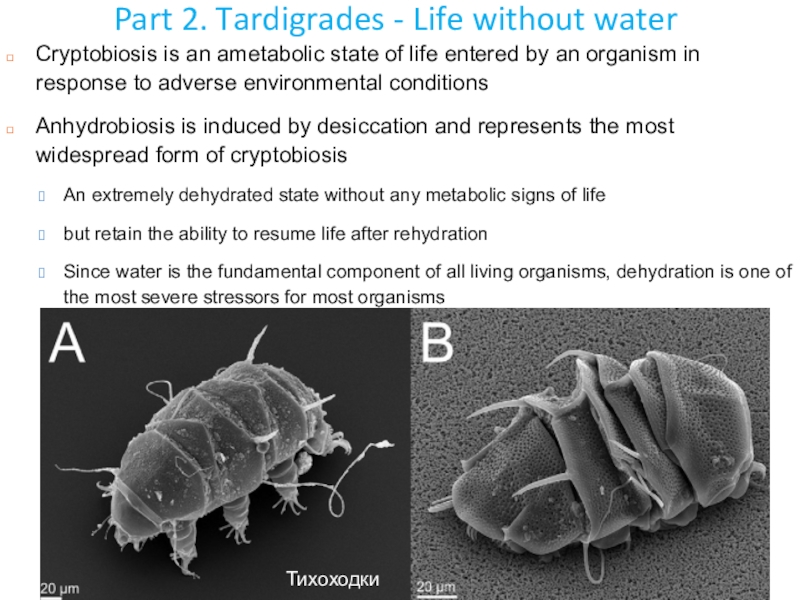
Слайд 40Part 2. Tardigrades - Life without water
Cryptobiosis is an
ametabolic state of life entered by an organism in response
to adverse environmental conditionsAnhydrobiosis is induced by desiccation and represents the most widespread form of cryptobiosis
An extremely dehydrated state without any metabolic signs of life
but retain the ability to resume life after rehydration
Since water is the fundamental component of all living organisms, dehydration is one of the most severe stressors for most organisms
Тихоходки
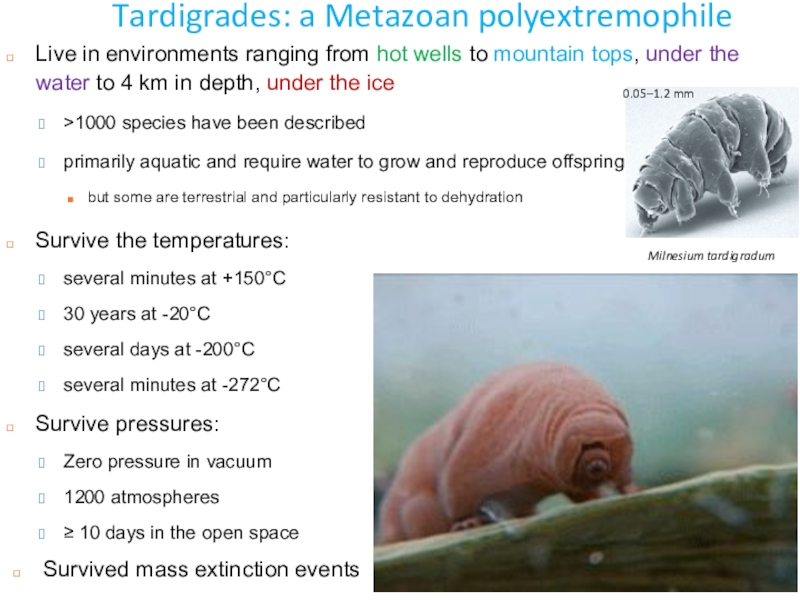
Слайд 41Tardigrades: a Metazoan polyextremophile
Milnesium tardigradum
Survive the temperatures:
several minutes at
+150°C
30 years at -20°C
several days at -200°C
several minutes at -272°C
Survive
pressures:Zero pressure in vacuum
1200 atmospheres
≥ 10 days in the open space
0.05–1.2 mm
Live in environments ranging from hot wells to mountain tops, under the water to 4 km in depth, under the ice
>1000 species have been described
primarily aquatic and require water to grow and reproduce offspring
but some are terrestrial and particularly resistant to dehydration
Survived mass extinction events
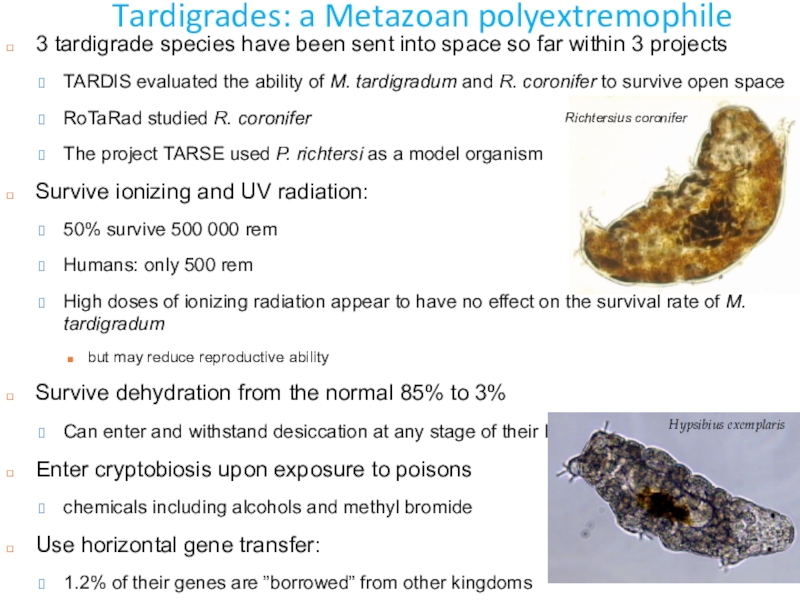
Слайд 42Tardigrades: a Metazoan polyextremophile
3 tardigrade species have been sent into
space so far within 3 projects
TARDIS evaluated the ability
of M. tardigradum and R. coronifer to survive open space RoTaRad studied R. coronifer
The project TARSE used P. richtersi as a model organism
Survive ionizing and UV radiation:
50% survive 500 000 rem
Humans: only 500 rem
High doses of ionizing radiation appear to have no effect on the survival rate of M. tardigradum
but may reduce reproductive ability
Survive dehydration from the normal 85% to 3%
Can enter and withstand desiccation at any stage of their life
Enter cryptobiosis upon exposure to poisons
chemicals including alcohols and methyl bromide
Use horizontal gene transfer:
1.2% of their genes are ”borrowed” from other kingdoms
Richtersius coronifer
Hypsibius exemplaris
Слайд 43Anhydrobiosis in tardigrades
Two main strategies to cope with water stress
are dehydration avoidance and tolerance
Most invertebrates use dehydration avoidance but
it is not always possibleDesiccation tolerance by anhydrobiosis
Anhydrobiotic organisms are found among unicellular organisms, invertebrates and plants
In response to adverse dry environmental condition semiterestrial tardigrades enter an anhydrobiotic state called a tun
which involves morphological changes such as retraction of body extremities
However, even in the active state they exhibit high tolerance to environmental stresses
Anhydrobiosis enables inhabiting harsh and extreme ecosystems
Notable intra- and interspecific differences in anhydrobiotic survival were found in several studies
The tun formation is an active process requiring metabolism
Therefore, tuns are formed only by active animals during desiccation
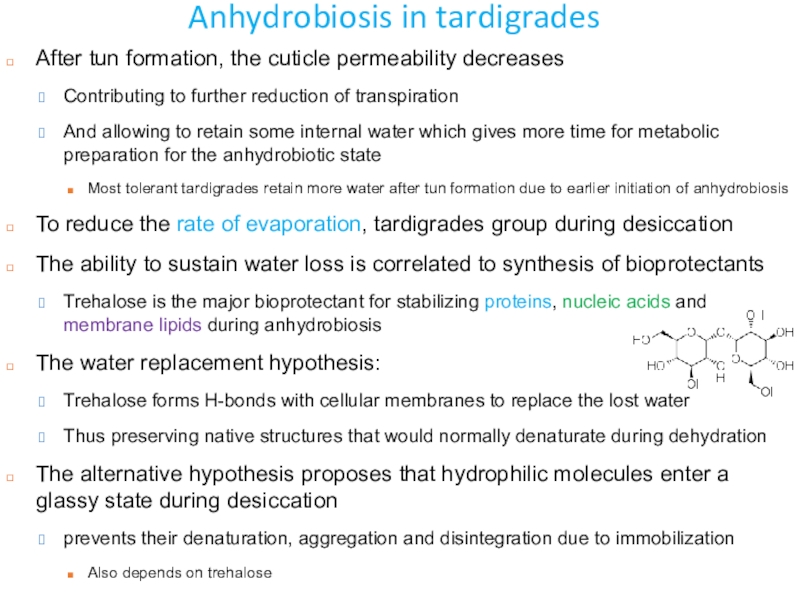
Слайд 44Anhydrobiosis in tardigrades
After tun formation, the cuticle permeability decreases
Contributing
to further reduction of transpiration
And allowing to retain some internal
water which gives more time for metabolic preparation for the anhydrobiotic state Most tolerant tardigrades retain more water after tun formation due to earlier initiation of anhydrobiosis
To reduce the rate of evaporation, tardigrades group during desiccation
The ability to sustain water loss is correlated to synthesis of bioprotectants
Trehalose is the major bioprotectant for stabilizing proteins, nucleic acids and membrane lipids during anhydrobiosis
The water replacement hypothesis:
Trehalose forms H-bonds with cellular membranes to replace the lost water
Thus preserving native structures that would normally denaturate during dehydration
The alternative hypothesis proposes that hydrophilic molecules enter a glassy state during desiccation
prevents their denaturation, aggregation and disintegration due to immobilization
Also depends on trehalose
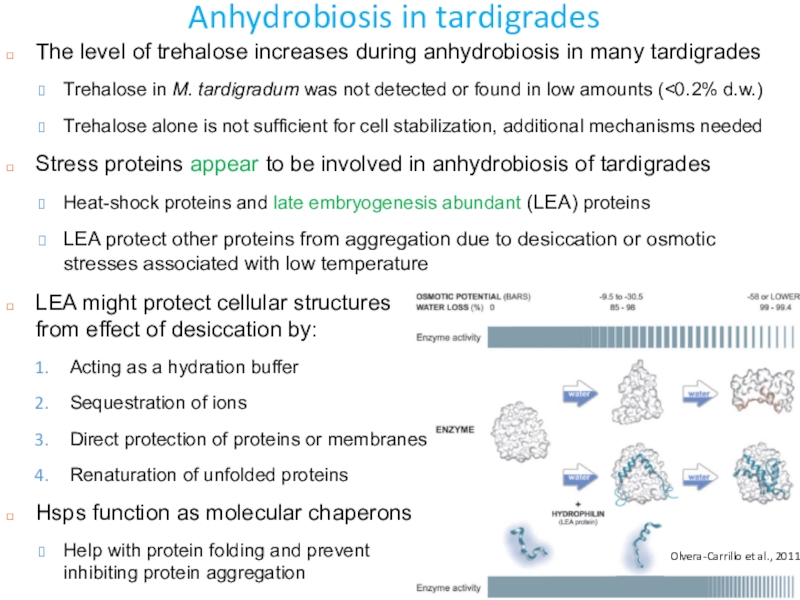
Слайд 45Anhydrobiosis in tardigrades
The level of trehalose increases during anhydrobiosis in
many tardigrades
Trehalose in M. tardigradum was not detected or found
in low amounts (<0.2% d.w.) Trehalose alone is not sufficient for cell stabilization, additional mechanisms needed
Stress proteins appear to be involved in anhydrobiosis of tardigrades
Heat-shock proteins and late embryogenesis abundant (LEA) proteins
LEA protect other proteins from aggregation due to desiccation or osmotic stresses associated with low temperature
LEA might protect cellular structures from effect of desiccation by:
Acting as a hydration buffer
Sequestration of ions
Direct protection of proteins or membranes
Renaturation of unfolded proteins
Hsps function as molecular chaperons
Help with protein folding and prevent inhibiting protein aggregation
Olvera-Carrillo et al., 2011
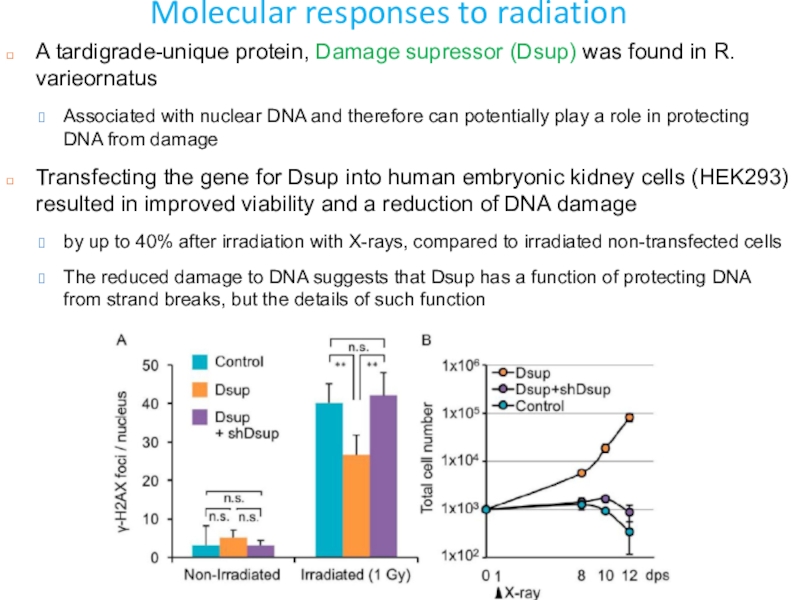
Слайд 46Molecular responses to radiation
A tardigrade-unique protein, Damage supressor (Dsup) was
found in R. varieornatus
Associated with nuclear DNA and therefore
can potentially play a role in protecting DNA from damageTransfecting the gene for Dsup into human embryonic kidney cells (HEK293) resulted in improved viability and a reduction of DNA damage
by up to 40% after irradiation with X-rays, compared to irradiated non-transfected cells
The reduced damage to DNA suggests that Dsup has a function of protecting DNA from strand breaks, but the details of such function
Слайд 47Longevity vs DNA damage
What are the consequences of desiccation on
longevity in tardigrades?
Three hypotheses about the effects of anhydrobiosis on
agingThe ‘‘Sleeping Beauty’’: the entire time in the anhydrobiotic state does not matter
The second accepts a partial discount of time spent in anhydrobiosis
The third model postulates that the anhydrobiotic organism registers the time spent in the anhydrobiotic state, resulting in a non-extended longevity
The internal clock of some rotifers seems to ignore the time spent in the anhydrobiotic state
But the nematode Panagrolaimus rigidus appears to count the time spent in the tun state
Shannon et al., 2005
Bdelloid rotifers
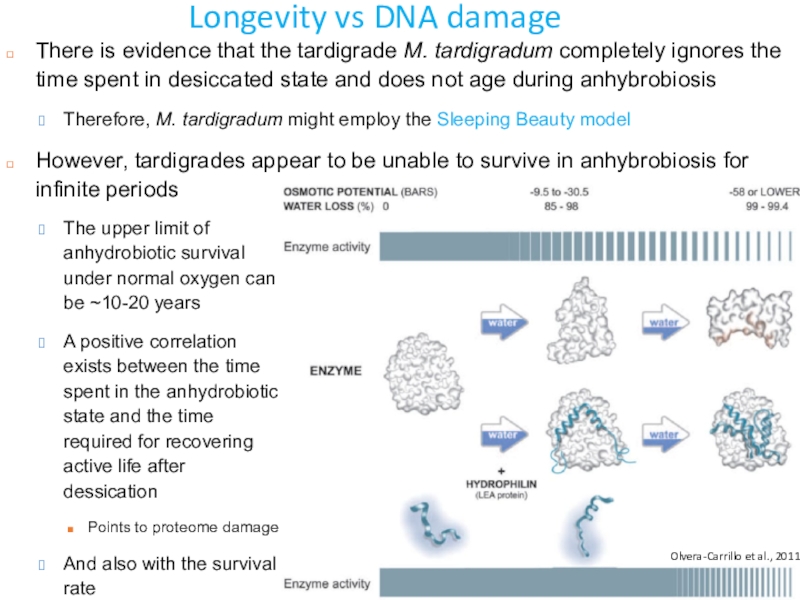
Слайд 48Longevity vs DNA damage
There is evidence that the tardigrade M.
tardigradum completely ignores the time spent in desiccated state and
does not age during anhybrobiosisTherefore, M. tardigradum might employ the Sleeping Beauty model
However, tardigrades appear to be unable to survive in anhybrobiosis for infinite periods
Olvera-Carrillo et al., 2011
The upper limit of anhydrobiotic survival under normal oxygen can be ~10-20 years
A positive correlation exists between the time spent in the anhydrobiotic state and the time required for recovering active life after dessication
Points to proteome damage
And also with the survival rate
Слайд 49Longevity vs DNA damage
Survival during anhydrobiosis could be limited by
accumulation of damaging effects of free radicals
ROS can be created
in response to increased ionic concentrations during desiccationcan degrade or destroy cellular and molecular structures, including DNA
ROS also inactivate the antioxidant enzymes as well as DNA repair enzymes
This cannot be repaired during anhydrobiosis
Consequently causes the death of anhydrobiotic organisms during the rehydration
The process of desiccation itself induces none or only minor DNA damages
Слайд 50Tardigrades in biomedical research
How genome integrity is maintained is a
central aspect in understanding radiation tolerance of tardigrades and other
cryptobiotic organismsAnd so also in the fields of human carcinogenesis and radiotherapy
But: genome vs proteome integrity?
Mechanisms that prevent damage and repair damage to DNA are probably involved in the desiccation/radiation tolerance of tardigrades
Study of these mechanisms might be relevant to cancer research
The Dsup study strongly points to potential benefits of collaboration between medical research and research on cryptobiotic animals
Слайд 51Tardigrades in biomedical research
Oxidative stress is a field where studies
on the tolerance of tardigrades have a high potential to
contribute to medical researchOxidative stress is a central aspect in the development of many diseases,
including, cancer, aging, diabetes, inflammation, Parkinson’s disease etc.
It is also an important aspect of radiotherapy because
The effect of low-linear energy transfer (LET) radiation treatment of cancer primarily relies on increased oxidative stress created by radiation-induced ROS
LEA proteins weakly interact with other macromolecules
to prevent undesirable aggregation of proteins or conformational changes of lipid membranes by acting as a ‘molecular shield’
LEA proteins can also act as ion-scavengers and anti-oxidants, but their precise functions are not completely understood
Introduction of LEA proteins to stress-sensitive human cells improved tolerability against various water stresses
such as dehydration, hyperosmotic treatment, and freezing
Some LEA proteins localize in subcellular compartments, e.g. mitochondria
protect macromolecules there