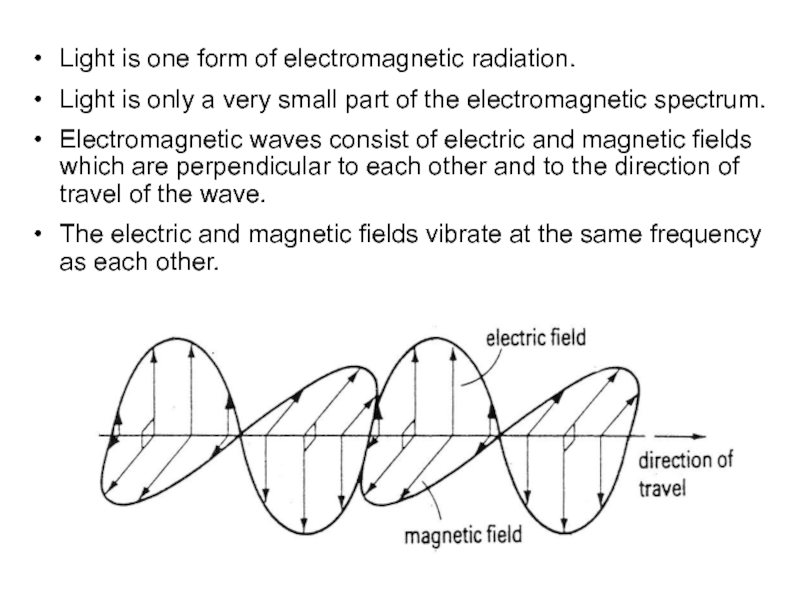
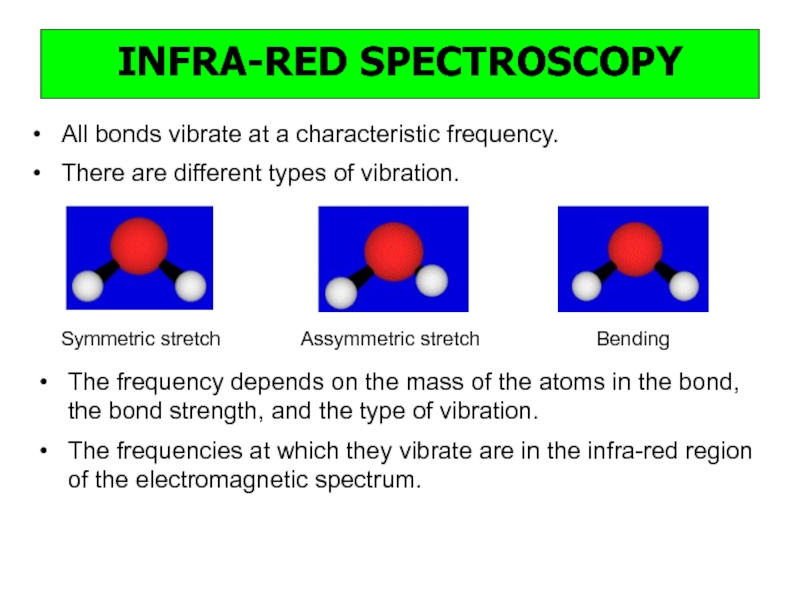
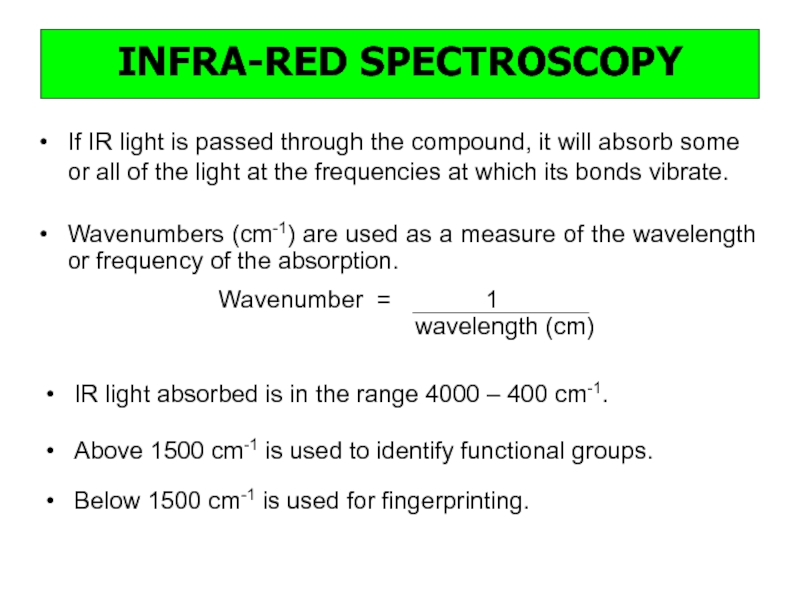
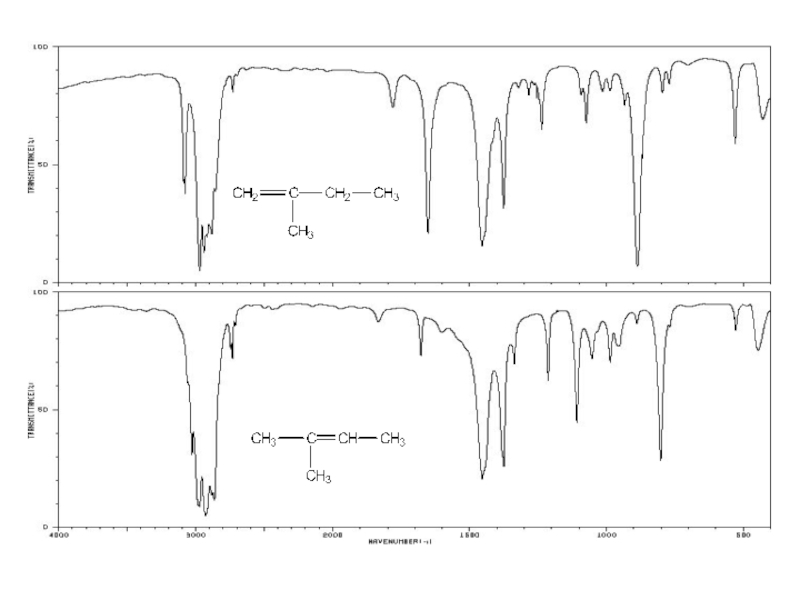
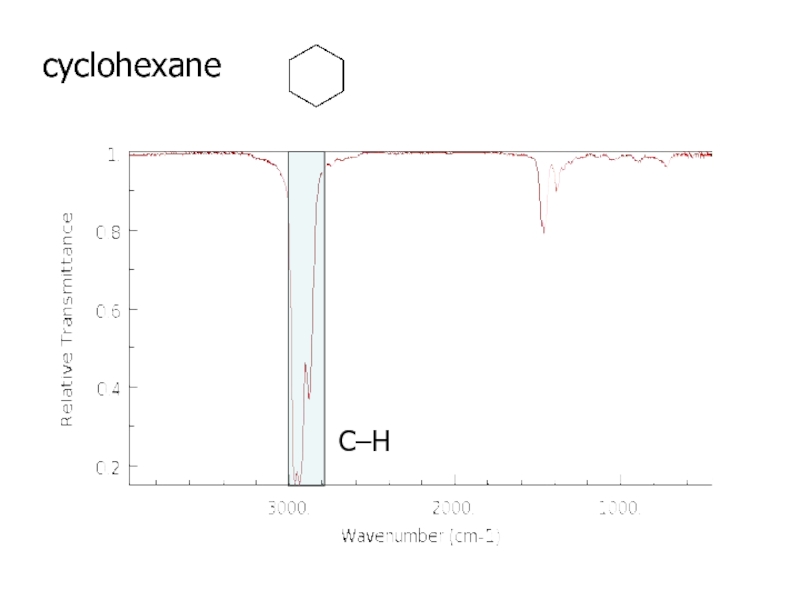
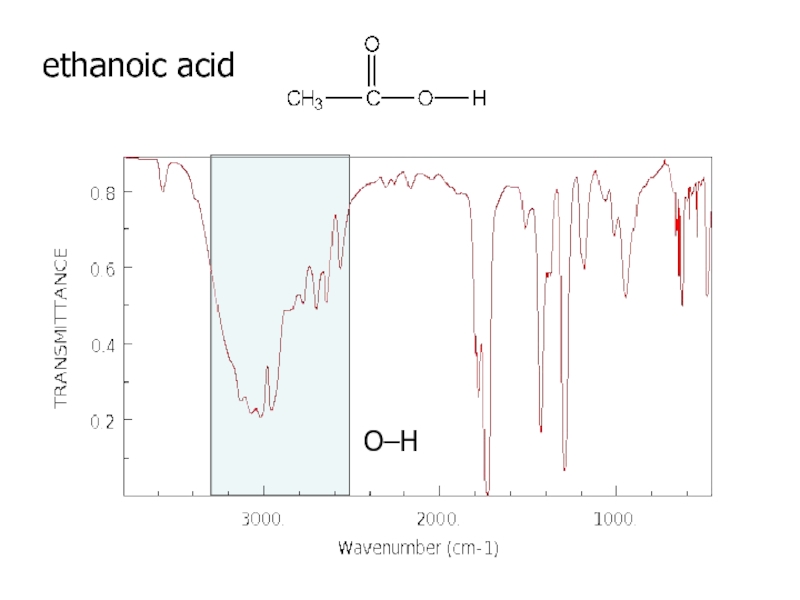
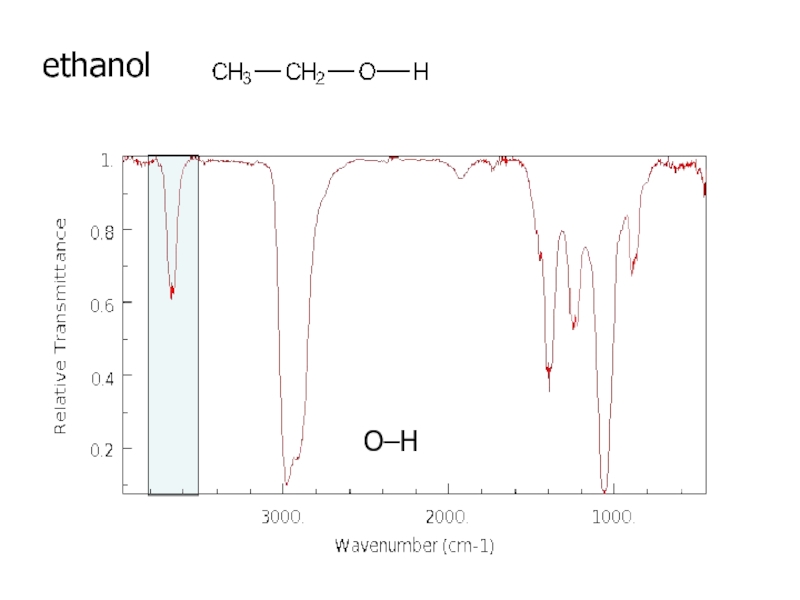
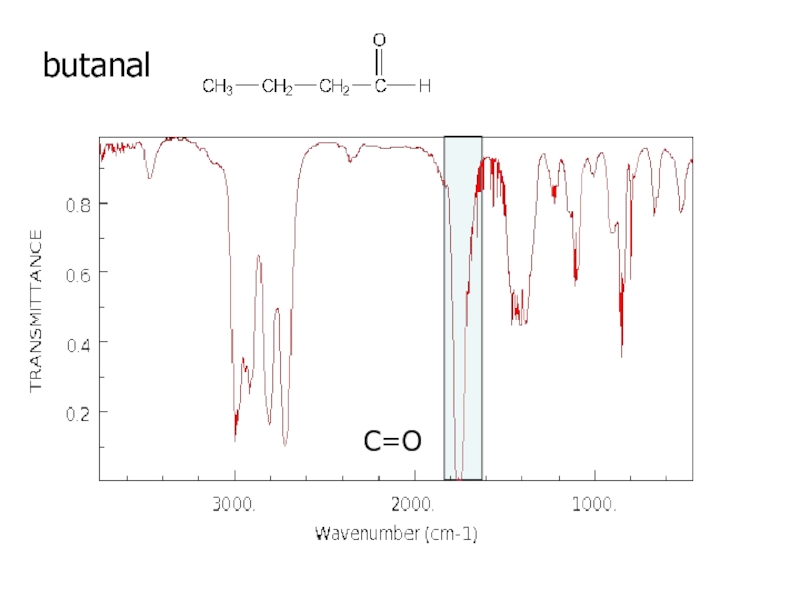
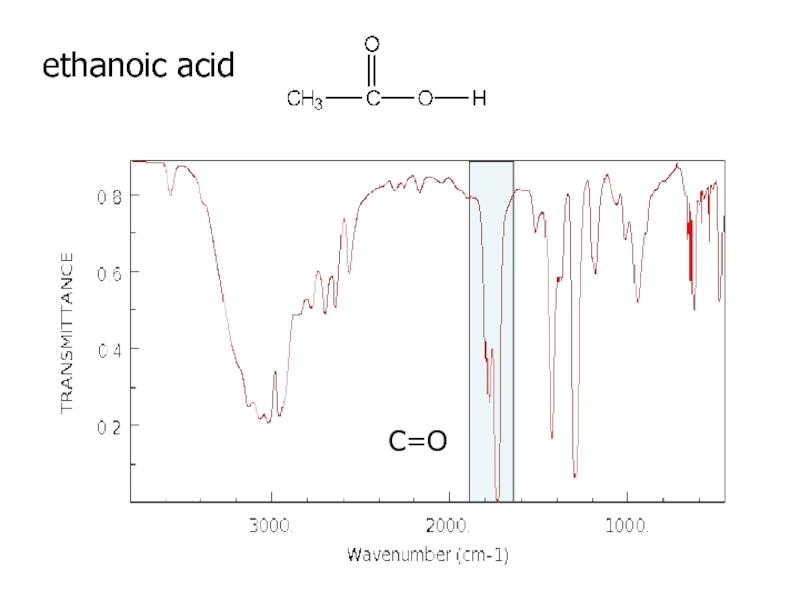
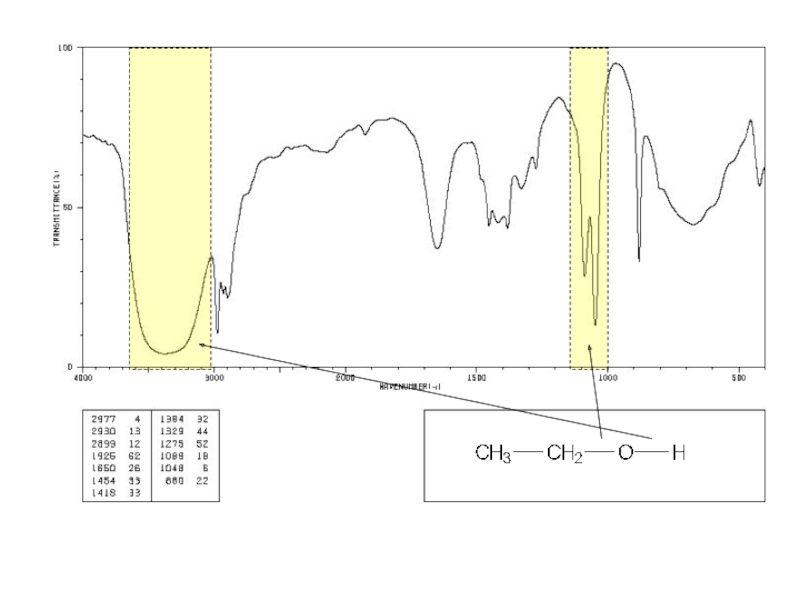
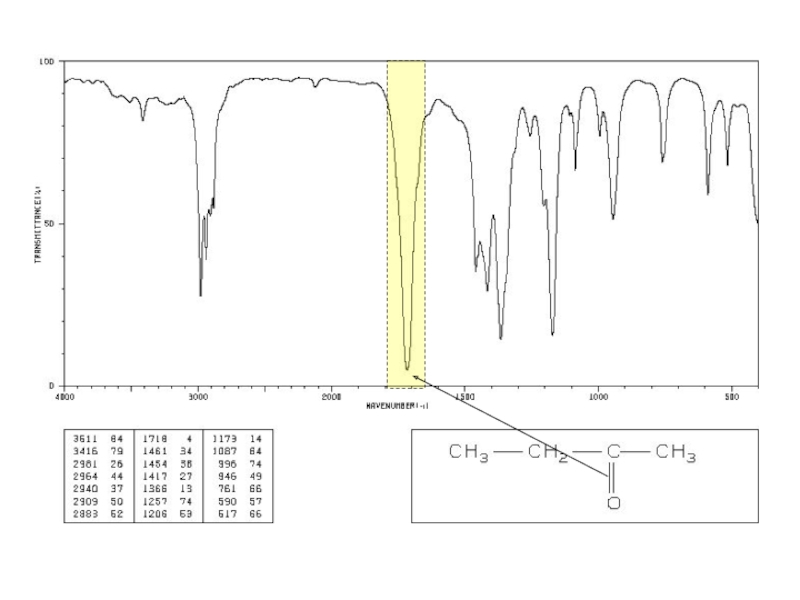
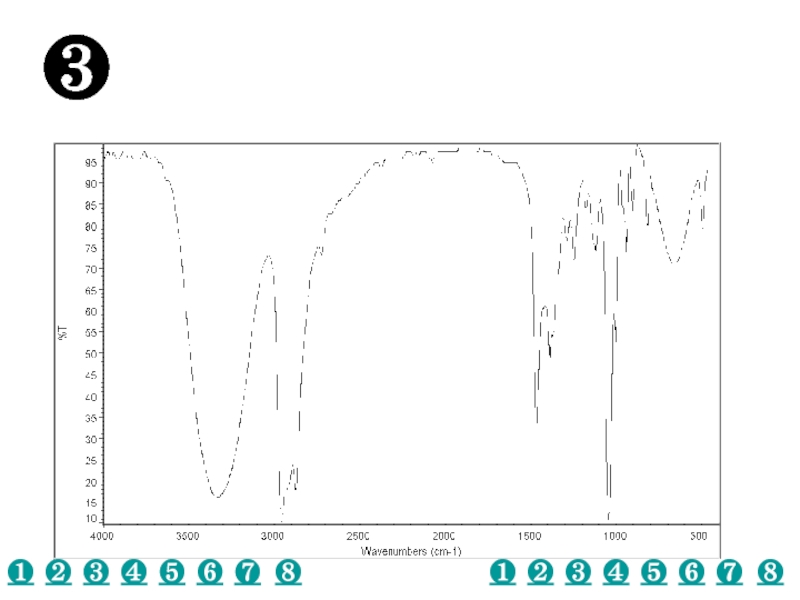
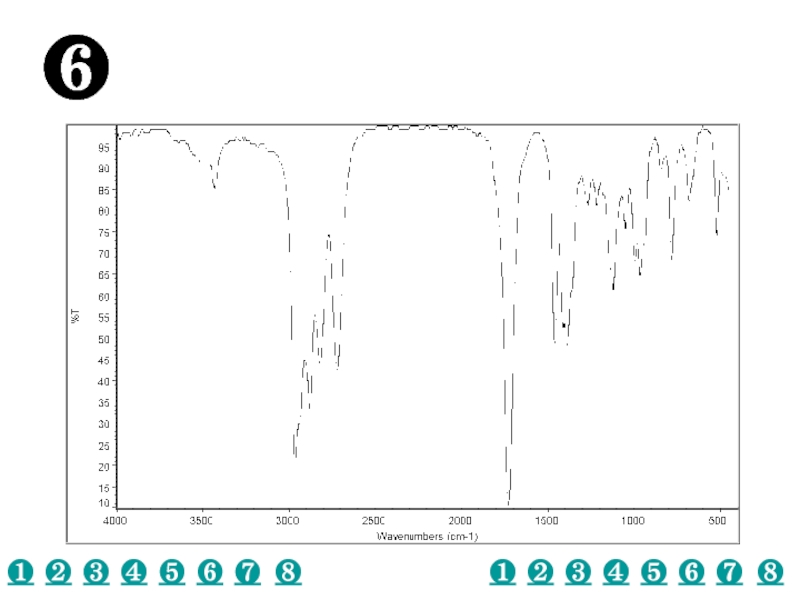
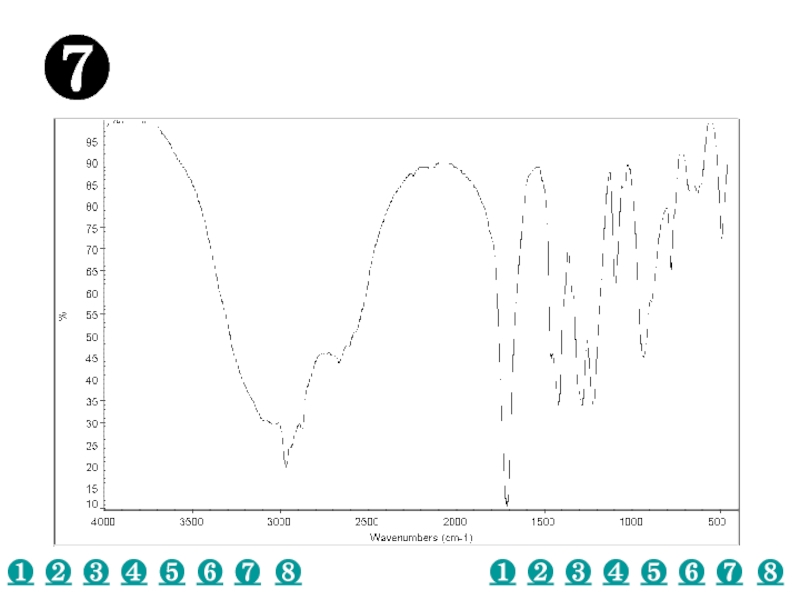
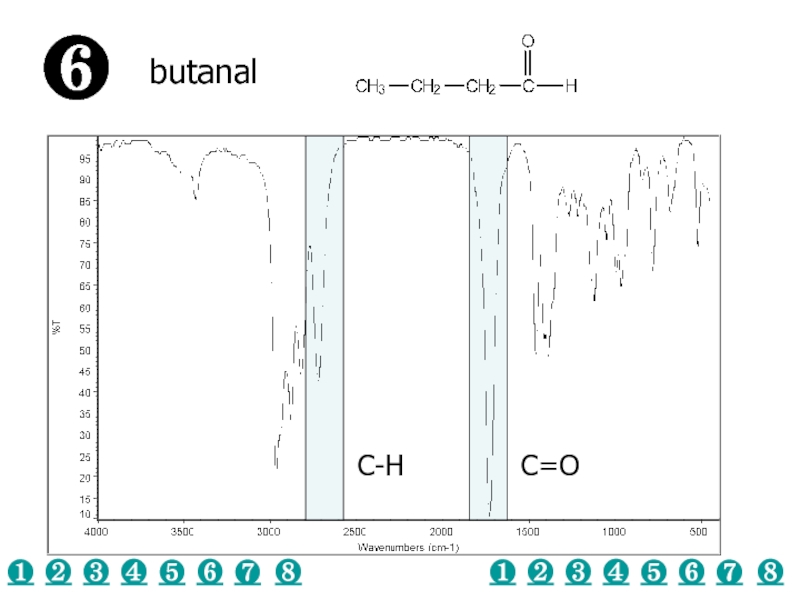
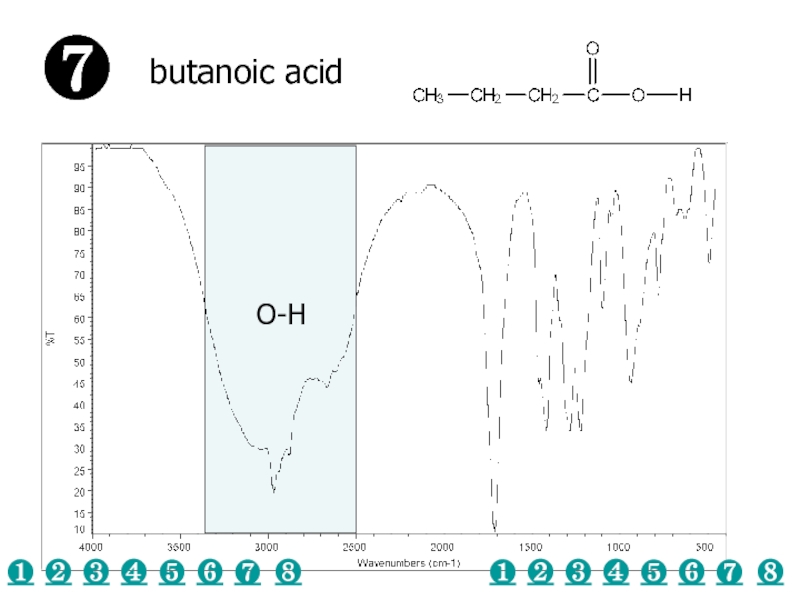
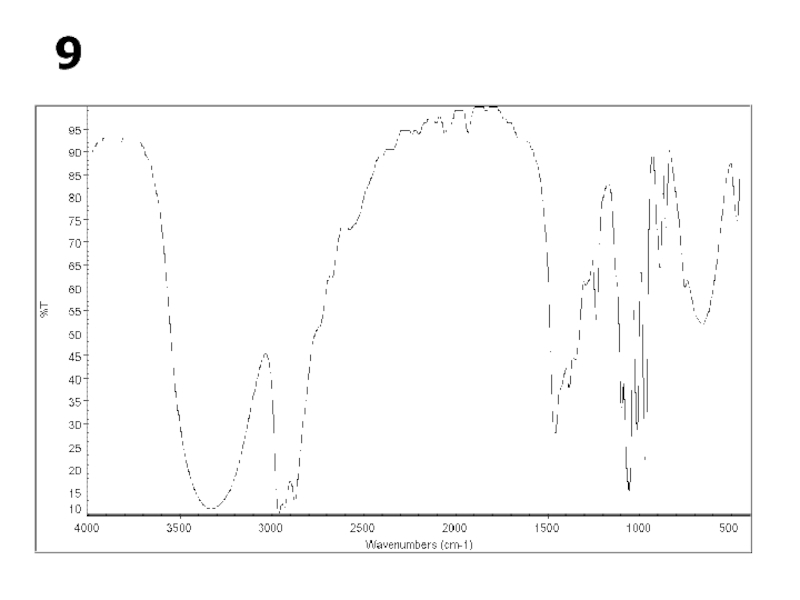
a very small part of the electromagnetic spectrum.
Electromagnetic waves consist
of electric and magnetic fields which are perpendicular to each other and to the direction of travel of the wave. The electric and magnetic fields vibrate at the same frequency as each other.