Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
L. 2 Methods of studies of cell structure
Содержание
- 1. L. 2 Methods of studies of cell structure
- 2. HomogenizationIf the cell is broken in gentle
- 3. Homogenization techniques can be divided into those
- 4. Cell lysis – critical steps Extraction of
- 5. Lysis methods Osmotic shock – 2 volumes
- 6. Lysis Method Cell Bomb – Place
- 7. Osmotic alterations Many organelles are easier
- 8. Mortars, Pestles Perhaps the most common
- 9. To obtain pure organelles, the cells must
- 10. With all forms of homogenization, the shear
- 11. BlendersFor molecular separations, mechanical blenders are often
- 12. Compression/Expansion For cellular material which is difficult
- 13. Buffer systems Main Components Buffer – a
- 14. Buffer systems Proteases are released as
- 15. Ultrasonification Ultrasonicators have been used with increasing
- 16. Stages to isolate cell structure
- 17. Compound MicroscopeIt is the most widely used
- 18. Electron MicroscopeIt is a sophisticated form of
- 19. Слайд 19
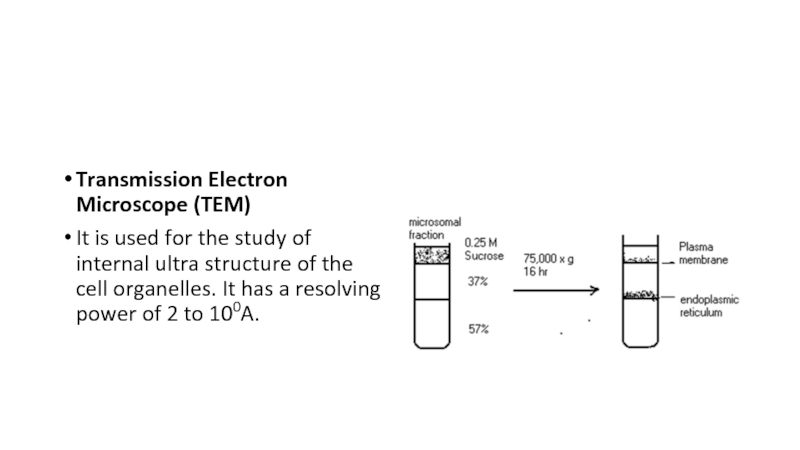
- 20. Transmission Electron Microscope (TEM)It is used for
- 21. Scanning Electron Microscope (SEM)It is used for
- 22. Principles and capacitiesThe types of signals produced
- 23. Слайд 23
- 24. Скачать презентанцию
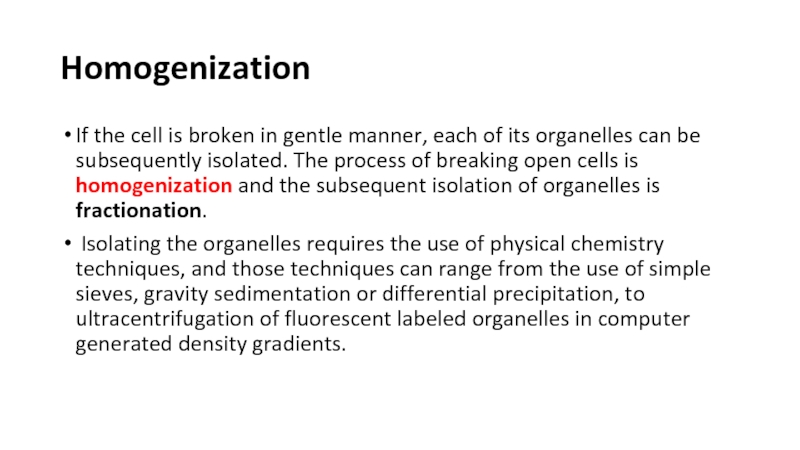
HomogenizationIf the cell is broken in gentle manner, each of its organelles can be subsequently isolated. The process of breaking open cells is homogenization and the subsequent isolation of organelles is
Слайды и текст этой презентации
Слайд 2Homogenization
If the cell is broken in gentle manner, each of
its organelles can be subsequently isolated. The process of breaking
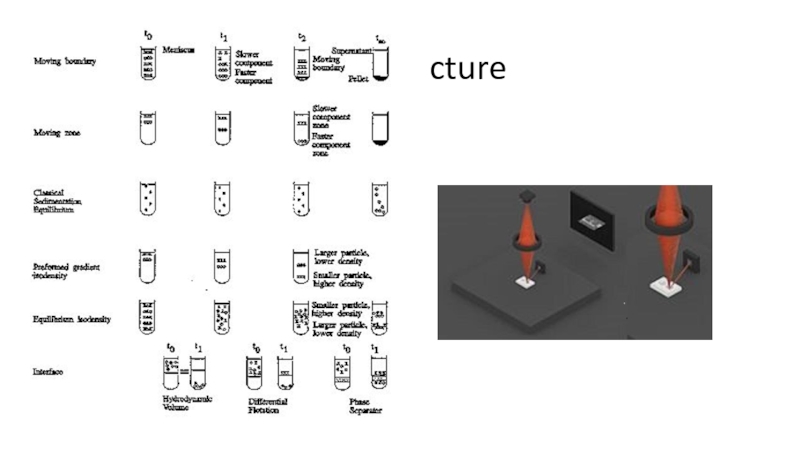
open cells is homogenization and the subsequent isolation of organelles is fractionation.Isolating the organelles requires the use of physical chemistry techniques, and those techniques can range from the use of simple sieves, gravity sedimentation or differential precipitation, to ultracentrifugation of fluorescent labeled organelles in computer generated density gradients.
Слайд 3Homogenization techniques can be divided into those brought about by
osmotic alteration of the media which cells are found in,
or those which require physical force to disrupt cell structure.
The physical means encompass use of mortars and pestles, blenders, compression and/or expansion, or ultrasonification.
Слайд 4Cell lysis
– critical steps
Extraction of structure from cells
includes specific and critical steps – a few conditions must
first be considered is your protein easily denatured? – temperature may be a factor, include thiols for disulfide stabilityIs protein hydrophobic? –to include detergents throughout isolation – know which detergents to use to avoid inhibition of your protein’s function
Is protein a target for proteases? Careful inclusion of specific protease inhibitors are needed
The next (downstream) step? Watch the ionic concentration or inclusion of various co-factors
• NaCl – for ion exchange EDTA for His tag purification
Слайд 5Lysis methods
Osmotic shock – 2 volumes of low osmotic
buffer (low salt) drives water into cell and bursts periplasmic
region – lowprotein but low protease
Enzymatic degradation – addition of lysozyme to cleave protein-sugar coating of cell wall, often combined with
another method (freeze thaw or mechanical sheering)
3. Freeze thaw – Quick freezing (LN2) and repeated thaw bursts cells as ice crystals forming in cells break open
membrane – used with shock or grinding
4. Abrasive Grinding – blend cell paste with sand or small glass beads – mechanically disrupts cells. Good for difficult to lyse cells and plant tissue
5. Sonication – used for moderate volumes of cells in suspension (5 – 50 ml). Cells are lysed by cavitation - quick
changes in pressure due to sound waves under tip.
Causes cells to swell and shrink and eventually burst
Temperature is a important. Keep on ice
French Press (valve-type processor) – Forces cell suspension through narrow needle valve under pressure
Lyses cells by sheering
Expensive set up but efficient for mid to large (not process) scale batches
Слайд 6Lysis Method
Cell Bomb – Place cells under high pressure
using nitrogen gas At high pressure, nitrogen gas becomes liquid
and freely diffuses into cell.Upon quick release of pressure, liquid inside of cell becomes gas and bursts cells
Expensive but effective for small to mid sized cultures
One step shopping – prepared solutions
Several companies have simple solutions which combine several components to lyse cells with minimal work.
• Bugbuster, B-PER, CellLytic, Qproteome, Bactozol
These can work very well, reduce need for sonication or other mechanical disruption and speed along lysis.
BUT for large scale cost can be significant
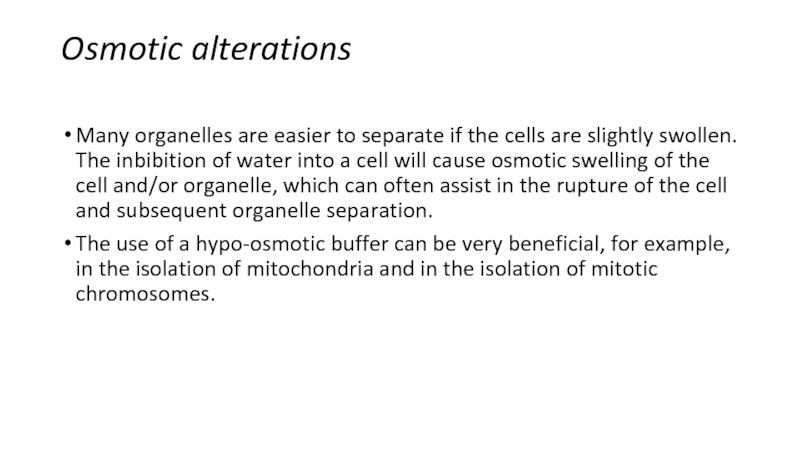
Слайд 7Osmotic alterations
Many organelles are easier to separate if the
cells are slightly swollen. The inbibition of water into a
cell will cause osmotic swelling of the cell and/or organelle, which can often assist in the rupture of the cell and subsequent organelle separation.The use of a hypo-osmotic buffer can be very beneficial, for example, in the isolation of mitochondria and in the isolation of mitotic chromosomes.
Слайд 8Mortars, Pestles
Perhaps the most common procedures use Ten Broeck
or Dounce homogenizers, both of which are glass mortar and
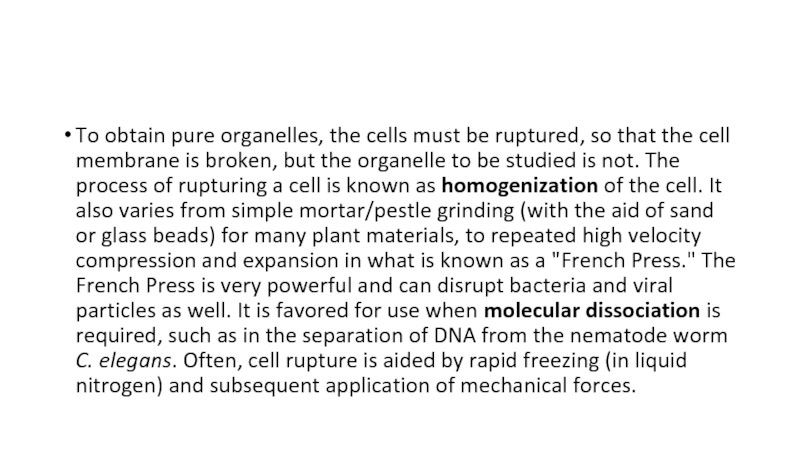
pestle arrangements with manufactured, controlled bore sizes. The addition of a motor driven teflon pestle creates the Potter-Elvijem homogenizer. Ultrasonification is a useful adjunct to this procedure, but is often sufficient by itself.Слайд 9To obtain pure organelles, the cells must be ruptured, so
that the cell membrane is broken, but the organelle to
be studied is not. The process of rupturing a cell is known as homogenization of the cell. It also varies from simple mortar/pestle grinding (with the aid of sand or glass beads) for many plant materials, to repeated high velocity compression and expansion in what is known as a "French Press." The French Press is very powerful and can disrupt bacteria and viral particles as well. It is favored for use when molecular dissociation is required, such as in the separation of DNA from the nematode worm C. elegans. Often, cell rupture is aided by rapid freezing (in liquid nitrogen) and subsequent application of mechanical forces.Слайд 10With all forms of homogenization, the shear force must be
carefully controlled. Too little and the organelles will not be
separated, too much and even the molecules can be broken.Слайд 11Blenders
For molecular separations, mechanical blenders are often used, varying in
sophistication from household blenders to high speed blenders with specially
designed blades and chambers (e.g. a Virtis Tissue Homogenizer). The mechanical procedures are augmented by various organic solvents (for phase separations) and/or detergents to assist the denaturation and separation of molecules (e.g. DNA from histones). When specific molecules are sought, care must be taken to inhibit powerful degradation enzymes (such as RNase when extracting RNA). This can be accomplished by subjecting the specimen to cold temperature, or by adding specific organic inhibitors (Diethylpyrocarbonate for RNase), or both.Слайд 12Compression/Expansion
For cellular material which is difficult to shear by
the above mentioned techniques (plant cells and bacteria), a device
known as a "French Press" is ocassionally used. This device forces a slurry of the cells through an orifice (opening) at very high pressures. The rapid expansion of the pressure from within literally "blows" the cells apart. While this technique is not often required, it is the only way to break open some materials. The units have capacities from 1 to 40 ml and can reach pressures of 20,000-40,000 pounds per square inch (psi).Слайд 13Buffer systems
Main Components
Buffer – a suitable buffer in the
pH range for purification and stability of your protein 10-50
mM in strength• Tris, Mops, HEPES are most commonly used
Salts – NaCL 25-200 mM, used to decrease weak ionic interactions between proteins and maintain ionic strength of
buffer
Detergents – Triton X-100 or Deoxycholate – helps solubilize cell membrane and maintain hydrophobic protein
structure
Chelators – EDTA (divalent cations – Fe+2…) EGTA (Calcium ion) 1 mM – reduces oxidation from free metals
EDTA will interfere with His-tag binding to Nickel column
Reducing Agents – Dithiolthreitol (DTT) or bmercaptoethanol (bME)
• At low concentration 1 – 10 mM act to maintain disulfide bridges
Other– Mg+2, ATP, substrates – help to maintain protein structure
DNA – Lysis will often denature histones resulting in an unraveling of genomic DNA – this results in a very viscous
solution –
mechanical sheering (needle sheering, sonication) or chemical treatment (DNAseI – 1.0 μg/ml) will be needed
Imidazole – may be used at low concentrations to prevent weak binding proteins loading onto Ni column
Слайд 14Buffer systems
Proteases are released as soon as the cell
is lysed and remain throughout most of the purification
Four
main classesSerine proteases (trypsin, chymotrypsin & elastase)
Cysteine proteases (papain, calpain and lysosomal cathespsins)
Aspartic proteases (pepsin and rennin)
Metallo-proteases thermolysisin & carboxypeptidaseA
Each class has a different set of inhibitors
Different organisms have different subsets of proteases. Thus it is important to have the right inhibitor(s) for your expression host
Many are soluble in DMSO or alcohol and have a short half life
Слайд 15Ultrasonification
Ultrasonicators have been used with increasing popularity to separate
organelles from cells, particularly from tissue culture cells. Light use
of an ultrasonic wave can readily remove cells from a tissue culture substrate (such as the culture flask). It can also be adjusted to merely separate cells, or to break open the plasma membrane and leave the internal organelles intactСлайд 17Compound Microscope
It is the most widely used instrument in the
field of biology. It is a device meant for magnifying
biological objects that cannot be seen with the naked human eye. It has two sets of lenses - objective and ocular - that magnify an object in two steps. A compound microscope can give a magnification of more than 1000 times. (The resolving power of a compound microscope is 0.25 mm).Слайд 18Electron Microscope
It is a sophisticated form of microscope, which can
provide a higher magnification of the object (up to 1,00,000
times or more). The electron microscope employs an electron beam emanating from a cathode, instead of light as in the compound microscope. It uses coils instead of lensesСлайд 20Transmission Electron Microscope (TEM)
It is used for the study of
internal ultra structure of the cell organelles. It has a
resolving power of 2 to 100A.Слайд 21Scanning Electron Microscope (SEM)
It is used for the study of
three-dimensional surface view of a given specimen. Its resolving power
is also around 2 to 100A. A scanning electron microscope (SEM) is a type of electron microscope that produces images of a sample by scanning it with a focused beam of electrons. The electrons interact with atoms in the sample, producing various signals that contain information about the sample's surface topography and composition. The electron beam is generally scanned in a raster scan pattern, and the beam's position is combined with the detected signal to produce an image. SEM can achieve resolution better than 1 nanometer. Specimens can be observed in high vacuum, in low vacuum, in wet conditions (in environmental SEM), and at a wide range of cryogenic or elevated temperatures.The most common SEM mode is detection of secondary electrons emitted by atoms excited by the electron beam. The number of secondary electrons that can be detected depends, among other things, on specimen topography. By scanning the sample and collecting the secondary electrons that are emitted using a special detector, an image displaying the topography of the surface is created.
Слайд 22Principles and capacitiesThe types of signals produced by an SEM
include secondary electrons (SE), reflected or back-scattered electrons (BSE), photons
of characteristic X-rays and light (cathodoluminescence) (CL), absorbed current (specimen current) and transmitted electrons. Secondary electron detectors are standard equipment in all SEMs, but it is rare that a single machine would have detectors for all other possible signals.The signals result from interactions of the electron beam with atoms at various depths within the sample. In the most common or standard detection mode, secondary electron imaging or SEI, the secondary electrons are emitted from very close to the specimen surface. Consequently, SEM can produce very high-resolution images of a sample surface, revealing details less than 1 nm in size. Back-scattered electrons (BSE) are beam electrons that are reflected from the sample by elastic scattering. They emerge from deeper locations within the specimen and consequently the resolution of BSE images is generally poorer than SE images. However, BSE are often used in analytical SEM along with the spectra made from the characteristic X-rays, because the intensity of the BSE signal is strongly related to the atomic number (Z) of the specimen. BSE images can provide information about the distribution of different elements in the sample. For the same reason, BSE imaging can image colloidal gold immuno-labels of 5 or 10 nm diameter, which would otherwise be difficult or impossible to detect in secondary electron images in biological specimens. Characteristic X-rays are emitted when the electron beam removes an inner shell electron from the sample, causing a higher-energy electron to fill the shell and release energy. These characteristic X-rays are used to identify the composition and measure the abundance of elements in the sample.
Due to the very narrow electron beam, SEM micrographs have a large depth of field yielding a characteristic three-dimensional appearance useful for understanding the surface structure of a sample. This is exemplified by the micrograph of pollen shown above. A wide range of magnifications is possible, from about 10 times (about equivalent to that of a powerful hand-lens) to more than 500,000 times, about 250 times the magnification limit of the best light microscopes.