Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Lecture № 5 Solutions. Equilibrium in solutions of strong and weak electrolytes
Содержание
- 1. Lecture № 5 Solutions. Equilibrium in solutions of strong and weak electrolytes
- 2. 1. Classification of solutions2. Solutions and concentration3.
- 3. Solvent — a substance that for convenience is treated
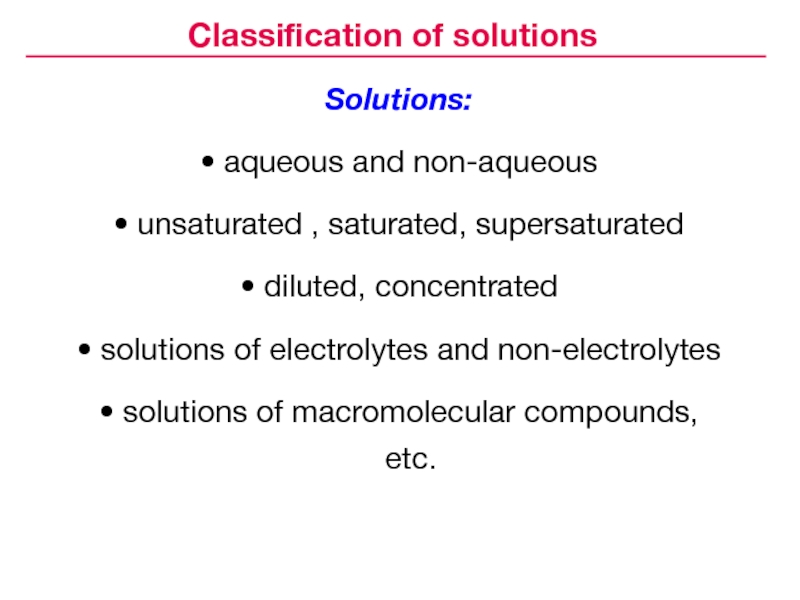
- 4. Classification of solutions
- 5. Solutions:aqueous and non-aqueousunsaturated , saturated, supersaturateddiluted, concentratedsolutions
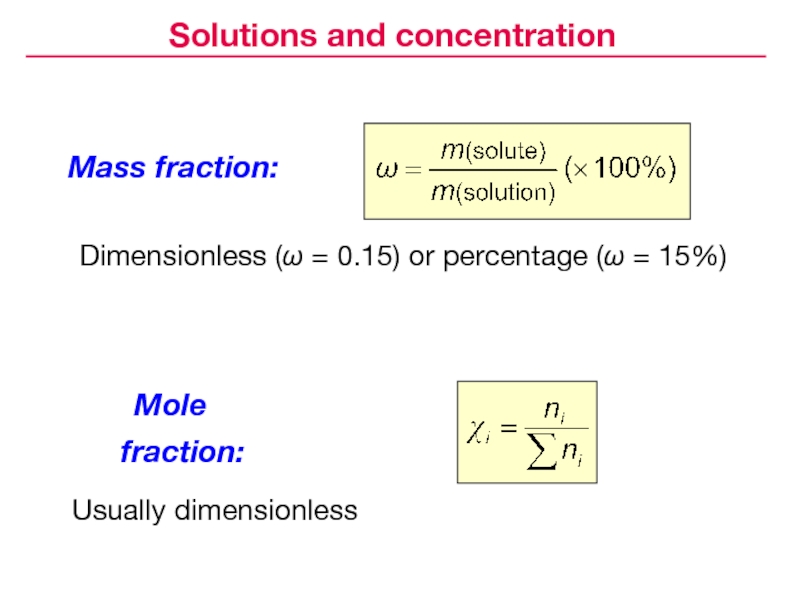
- 6. Mass fraction:Dimensionless (ω = 0.15) or percentage (ω = 15%)Mole fraction:Usually dimensionlessSolutions and concentration
- 7. Amount concentration (molarity):Mole per liter (mol/L or
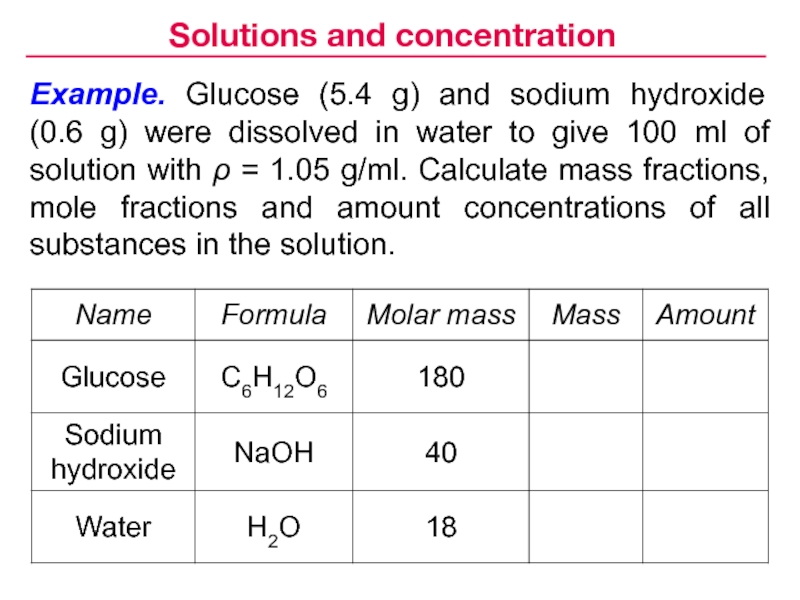
- 8. Example. Glucose (5.4 g) and sodium hydroxide
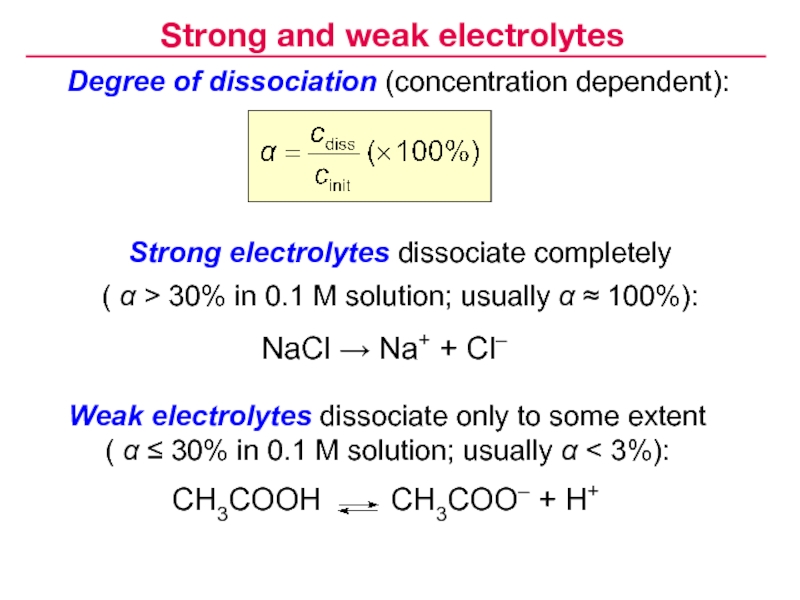
- 9. Degree of dissociation (concentration dependent):CH3COOH
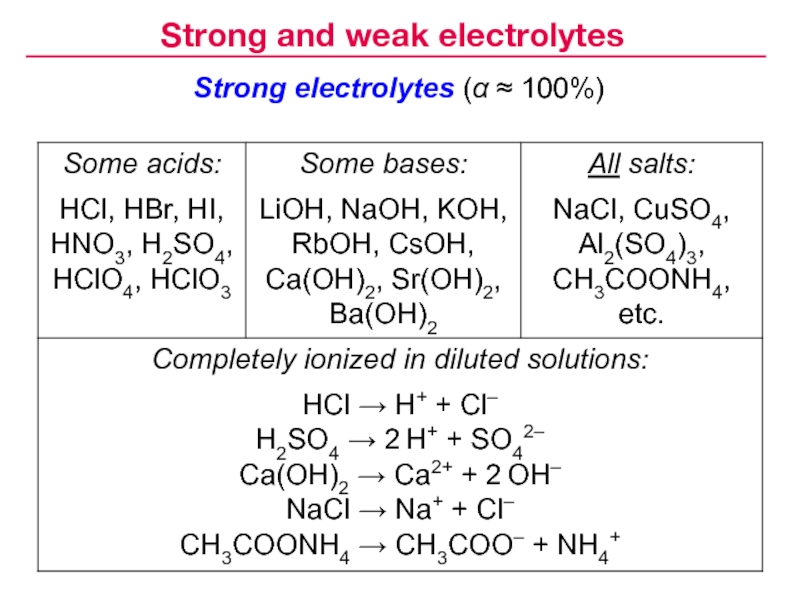
- 10. Strong electrolytes (α ≈ 100%)Strong and weak electrolytes
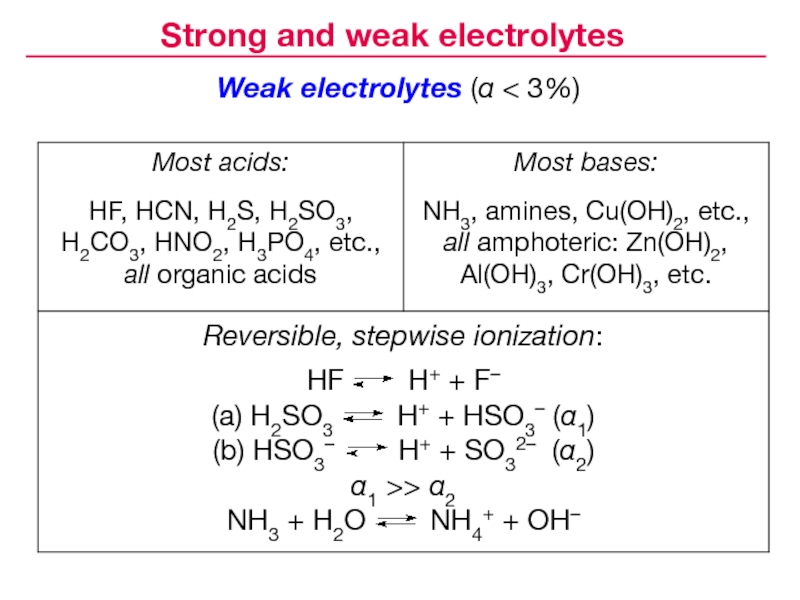
- 11. Weak electrolytes (α < 3%)Strong and weak electrolytes
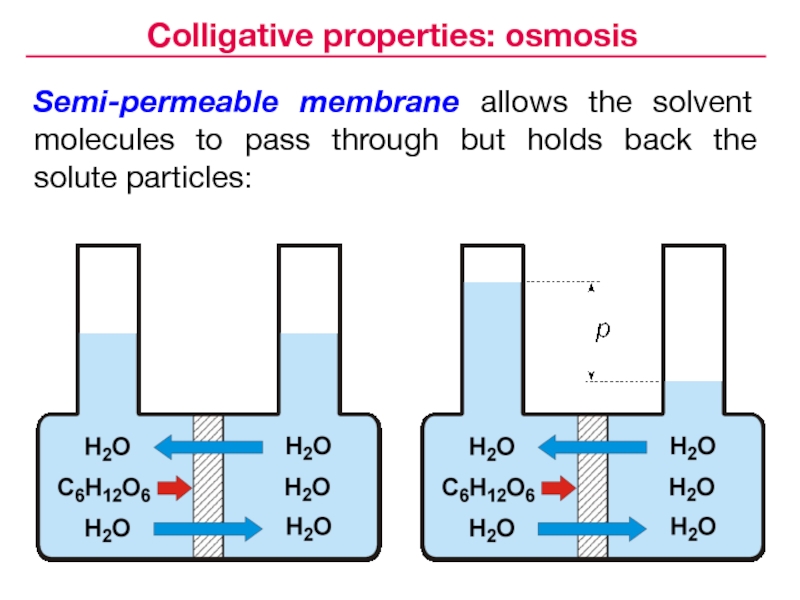
- 12. Semi-permeable membrane allows the solvent molecules to
- 13. Osmotic pressure — an excess pressure required
- 14. Isotonic coefficient, or van't Hoff factor (i)
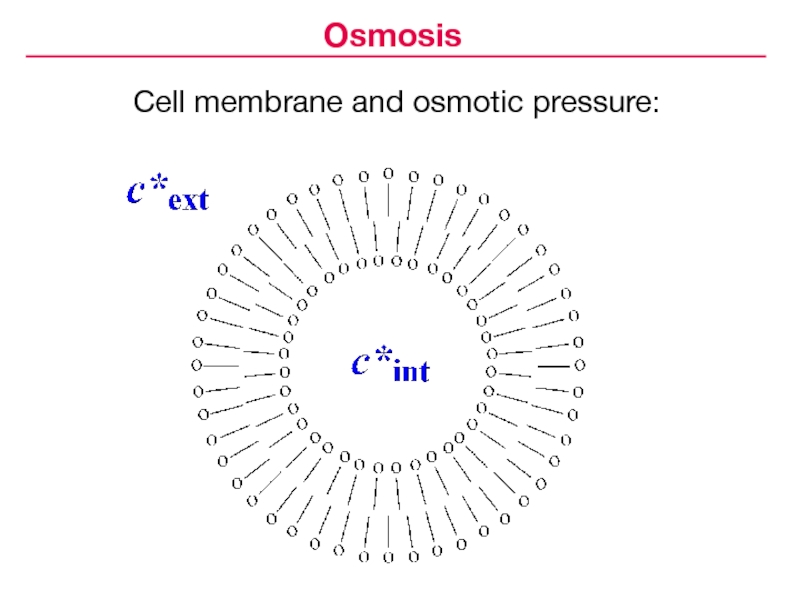
- 15. Cell membrane and osmotic pressure:Osmosis
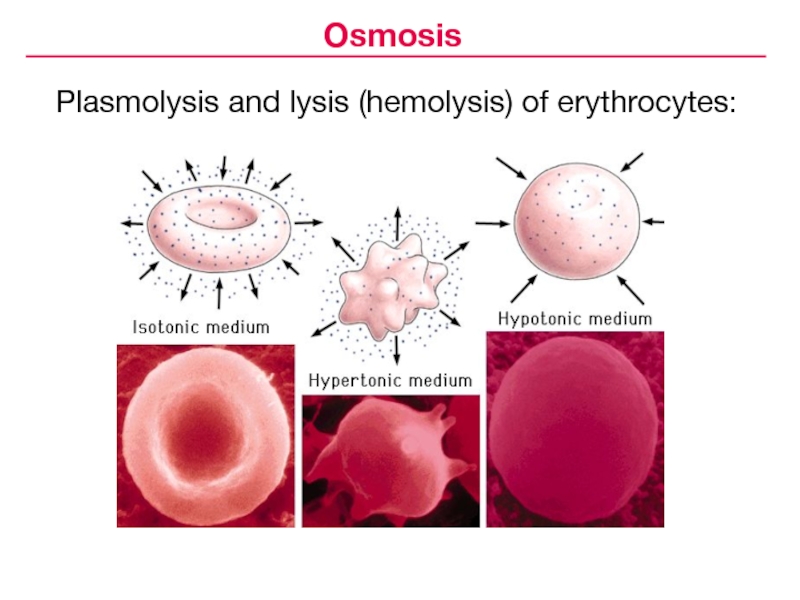
- 16. Plasmolysis and lysis (hemolysis) of erythrocytes:Osmosis
- 17. Water is a weak electrolyte: H2O
- 18. in pure water pH = pOH =
- 19. – lg[H+] – lg[OH–] = – lg(1.0 • 10–14)
- 20. Strong acids:HX → H+ + X–H2O
- 21. Example: The pH of 0.05 M solution
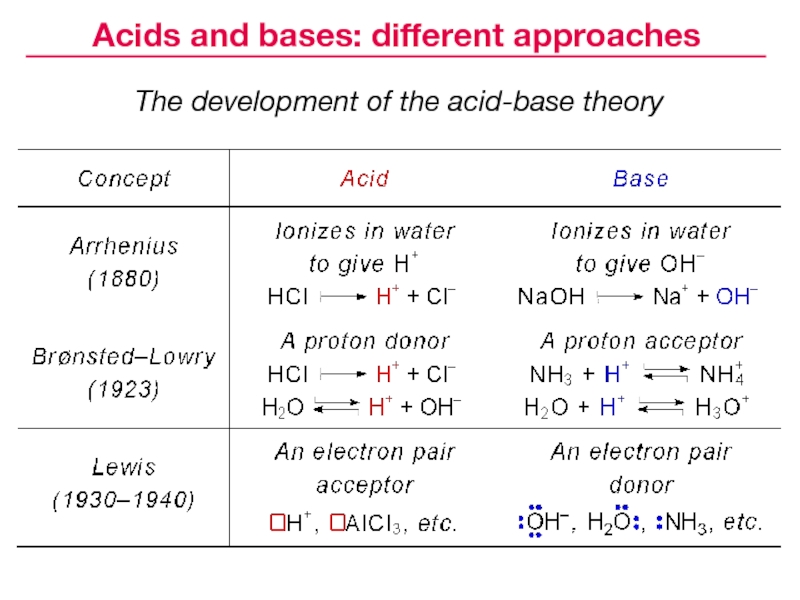
- 22. The development of the acid-base theoryAcids and bases: different approaches
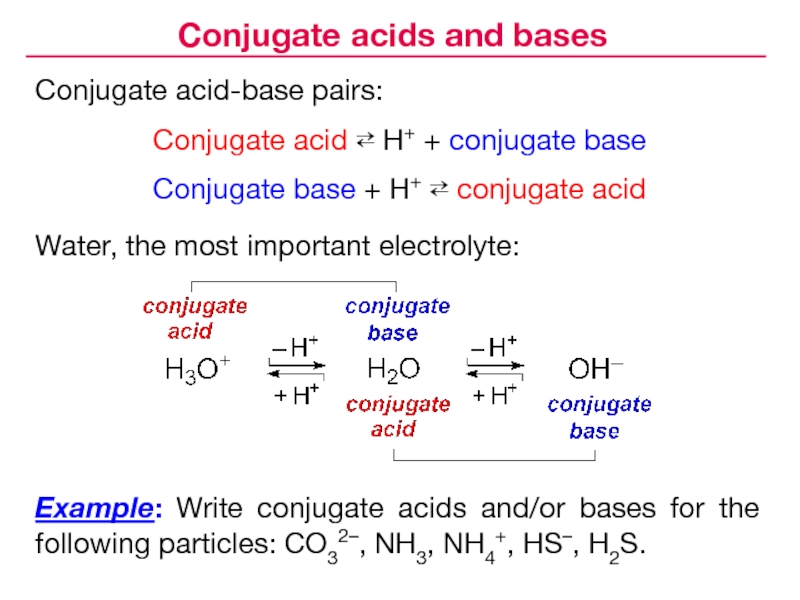
- 23. Conjugate acid-base pairs:Conjugate acid ⇄ H+ +
- 24. Acid-base equilibria in aqueous solutions[H+] < C(HA)[OH–] < C(B)Weak acids and bases
- 25. Strong acid:HCl → H+ + Cl–H2O
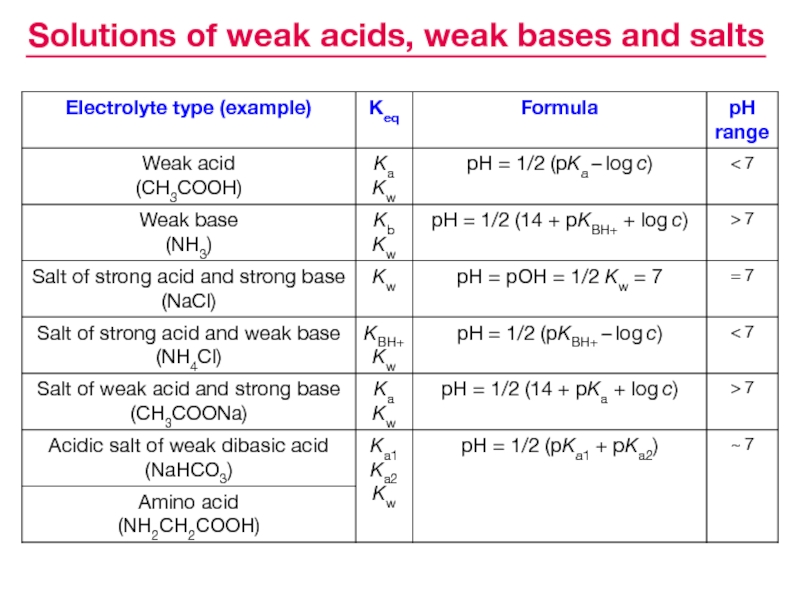
- 26. Solutions of weak acids, weak bases and salts
- 27. Слайд 27
- 28. Скачать презентанцию
Слайды и текст этой презентации
Слайд 21. Classification of solutions
2. Solutions and concentration
3. Strong and weak
electrolytes
4. Colligative properties: osmosis
5. Osmosis
6. The pH concept
Context
7. Strong acids
and bases8. Acids and bases: different approaches
9. Conjugate acids and bases
11. pH in solutions of strong and weak acids
10. Weak acids and bases
12. Solutions of weak acids, weak bases and salts
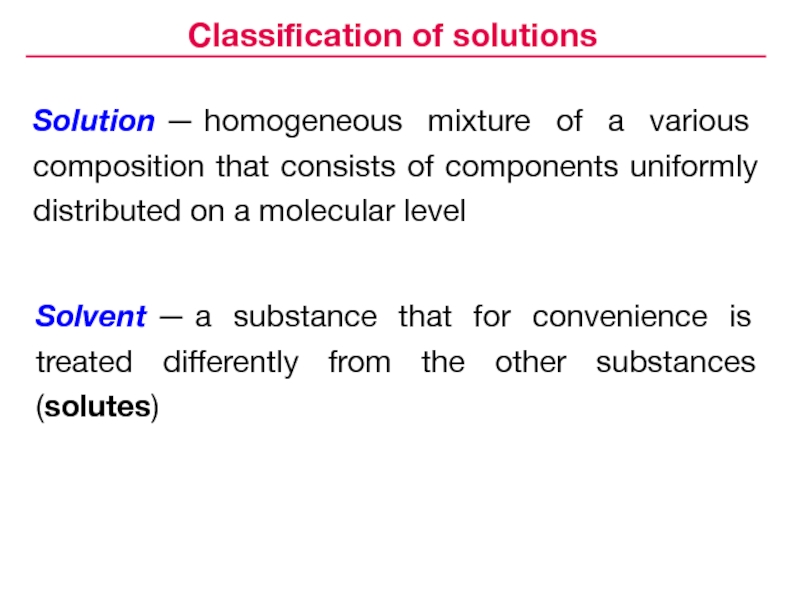
Слайд 3Solvent — a substance that for convenience is treated differently from the
other substances (solutes)
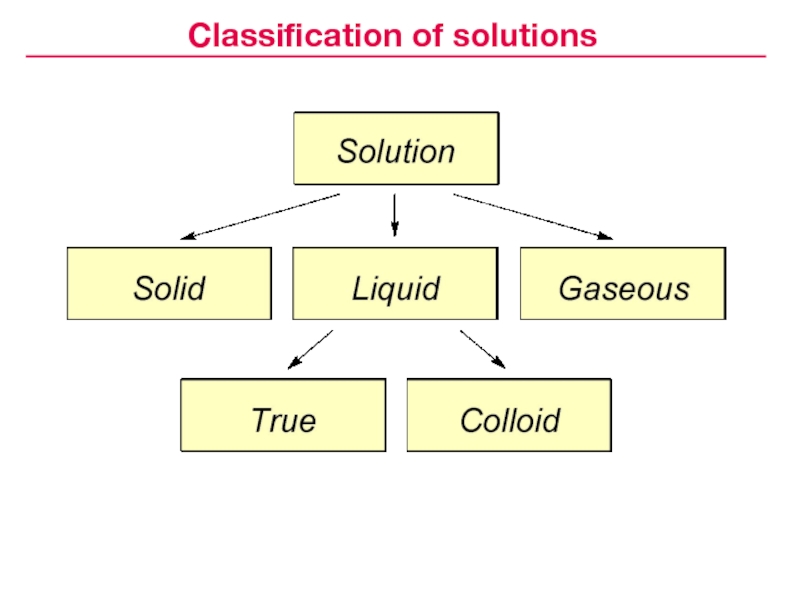
Classification of solutions
Solution — homogeneous mixture of a various
composition that consists of components uniformly distributed on a molecular levelСлайд 5Solutions:
aqueous and non-aqueous
unsaturated , saturated, supersaturated
diluted, concentrated
solutions of electrolytes and
non-electrolytes
solutions of macromolecular compounds, etc.
Classification of solutions
Слайд 6Mass fraction:
Dimensionless (ω = 0.15) or percentage (ω = 15%)
Mole
fraction:
Usually dimensionless
Solutions and concentration
Слайд 7Amount concentration
(molarity):
Mole per liter (mol/L or M);
Molality:
Mole per kg;
for diluted
solutions b[mol/kg] ≈ c[mol/L]
Solutions and concentration
Слайд 8Example. Glucose (5.4 g) and sodium hydroxide (0.6 g) were
dissolved in water to give 100 ml of solution with
ρ = 1.05 g/ml. Calculate mass fractions, mole fractions and amount concentrations of all substances in the solution.Solutions and concentration
Слайд 9Degree of dissociation (concentration dependent):
CH3COOH CH3COO– +
H+
Strong electrolytes dissociate completely
( α > 30% in 0.1 M
solution; usually α ≈ 100%):NaCl → Na+ + Cl–
Weak electrolytes dissociate only to some extent
( α ≤ 30% in 0.1 M solution; usually α < 3%):
Strong and weak electrolytes
Слайд 12Semi-permeable membrane allows the solvent molecules to pass through but
holds back the solute particles:
Colligative properties: osmosis
Слайд 13Osmotic pressure — an excess pressure required to maintain osmotic
equilibrium between a solution and the pure solvent separated by
a membrane permeable only to the solvent:Osmolarity (cosm) — the total molar concentration of all solvated particles of the solutes in the solution:
Osmosis
Слайд 14Isotonic coefficient, or van't Hoff factor (i) — the number
of moles of particles (ions and/or undissociated molecules) per mole
of solute:Osmosis
Слайд 17Water is a weak electrolyte:
H2O H+
+ OH–
aA + bB cC
+ dDH2O H+ + OH–
Kdiss (H2O)= 1.8 • 10–16 at 25 °C
ceq(X) = [X]
c(H2O) ≈ [H2O] = = 55.6 mol/L
The pH concept
Слайд 20Strong acids:
HX → H+ + X–
H2O H+
+ OH–
[H+] > [OH–]
pH < 7
Strong bases:
MOH → M+ + OH–
H2O
H+ + OH–[H+] < [OH–]
pH > 7
The pH concept
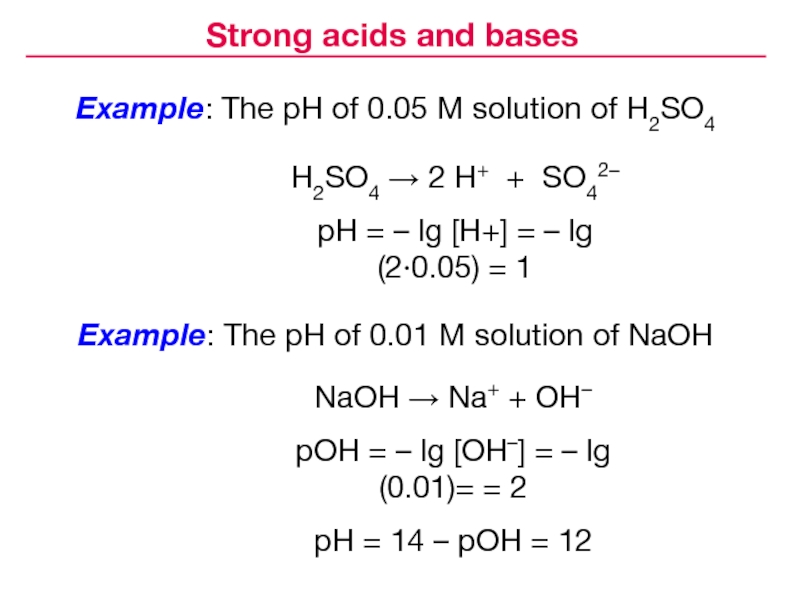
Слайд 21Example: The pH of 0.05 M solution of H2SO4
Example: The
pH of 0.01 M solution of NaOH
Strong acids and bases
H2SO4
2 H+ + SO42–pH = – lg [H+] = – lg (2·0.05) = 1
NaOH Na+ + OH–
pOH = – lg [OH–] = – lg (0.01)= = 2
pH = 14 – pOH = 12
Слайд 23Conjugate acid-base pairs:
Conjugate acid ⇄ H+ + conjugate base
Conjugate base
+ H+ ⇄ conjugate acid
Example: Write conjugate acids and/or bases
for the following particles: CO32–, NH3, NH4+, HS–, H2S.Water, the most important electrolyte:
Conjugate acids and bases
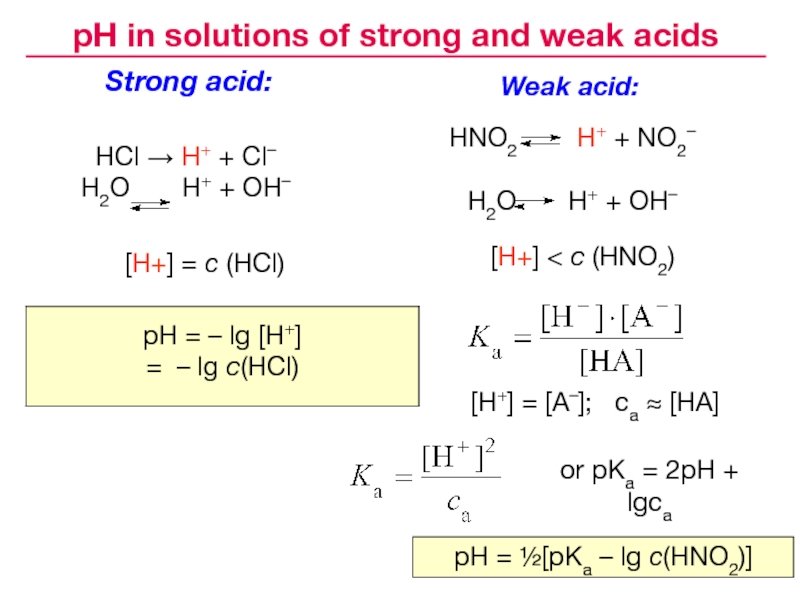
Слайд 25Strong acid:
HCl → H+ + Cl–
H2O H+
+ OH–
HNO2 H+ + NO2–
H2O
H+ + OH–Weak acid:
[H+] < c (HNO2)
[H+] = c (HCl)
[H+] = [A–]; ca [HA]
or pKa = 2pH + lgca
pH = ½[pKa – lg c(HNO2)]
pH in solutions of strong and weak acids


![Lecture № 5 Solutions. Equilibrium in solutions of strong and weak electrolytes Amount concentration (molarity):Mole per liter (mol/L or M);Molality:Mole per kg;for diluted Amount concentration (molarity):Mole per liter (mol/L or M);Molality:Mole per kg;for diluted solutions b[mol/kg] ≈ c[mol/L]Solutions and concentration](/img/thumbs/74327292dfae9ec90bb7a673b1bff54e-800x.jpg)



![Lecture № 5 Solutions. Equilibrium in solutions of strong and weak electrolytes in pure water pH = pOH = 7– lg[H+] – lg[OH–] in pure water pH = pOH = 7– lg[H+] – lg[OH–] = – lg(1.0 • 10–14) = 14The pH](/img/thumbs/c332fc41a105ed458d2daec20ba39036-800x.jpg)
![Lecture № 5 Solutions. Equilibrium in solutions of strong and weak electrolytes – lg[H+] – lg[OH–] = – lg(1.0 • 10–14) = 14in pure water – lg[H+] – lg[OH–] = – lg(1.0 • 10–14) = 14in pure water pH = pOH = 7The pH](/img/tmb/3/294550/961a10262739f17c2349568854f3379c-800x.jpg)
![Lecture № 5 Solutions. Equilibrium in solutions of strong and weak electrolytes Strong acids:HX → H+ + X–H2O H+ + OH–[H+] > [OH–] pH Strong acids:HX → H+ + X–H2O H+ + OH–[H+] > [OH–] pH < 7Strong bases:MOH →](/img/thumbs/df8208a00de0ad35c0f41ad014bdad1e-800x.jpg)
![Lecture № 5 Solutions. Equilibrium in solutions of strong and weak electrolytes Acid-base equilibria in aqueous solutions[H+] < C(HA)[OH–] < C(B)Weak acids and bases Acid-base equilibria in aqueous solutions[H+] < C(HA)[OH–] < C(B)Weak acids and bases](/img/tmb/3/294550/224e24ec208bb3b475e426a1997cec05-800x.jpg)