Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Lectures 8 and 9: Bioenergetics and the Regulation of Glycolysis Essential Cell
Содержание
- 1. Lectures 8 and 9: Bioenergetics and the Regulation of Glycolysis Essential Cell
- 2. Energy Flow in Biota:Light energy from the
- 3. This reaction yields energy due to breakingof
- 4. Слайд 4
- 5. Now consider glycolysis! C6H12O6 + 6 O2
- 6. Reactions in cells that make new macro-molecules
- 7. How do we transfer the energy yield
- 8. ΔGO = - 7 Kilocalories/moleHigh Energy Compounds Transfer Energy
- 9. Polymerization of RNA requires a high energy intermediate formed by ATP hydrolysis
- 10. A consequence of a chemical reaction beingfavorable
- 11. Слайд 11
- 12. Слайд 12
- 13. Or the simplest example:Assume that the second
- 14. Glycolysis isbroken upinto ANAEROBIC GLYCOLYSIS inthe cytoplasm(10
- 15. The highly favoredreactions are steps1,3 and 10.
- 16. The highly energetically favorable steps in anaerobic
- 17. ATP and ADP are important allosteric regulators!Speed
- 18. Enzymes go through the catalysis cycle shown
- 19. Technique: Measuring Enzyme Reaction Velocity: One can
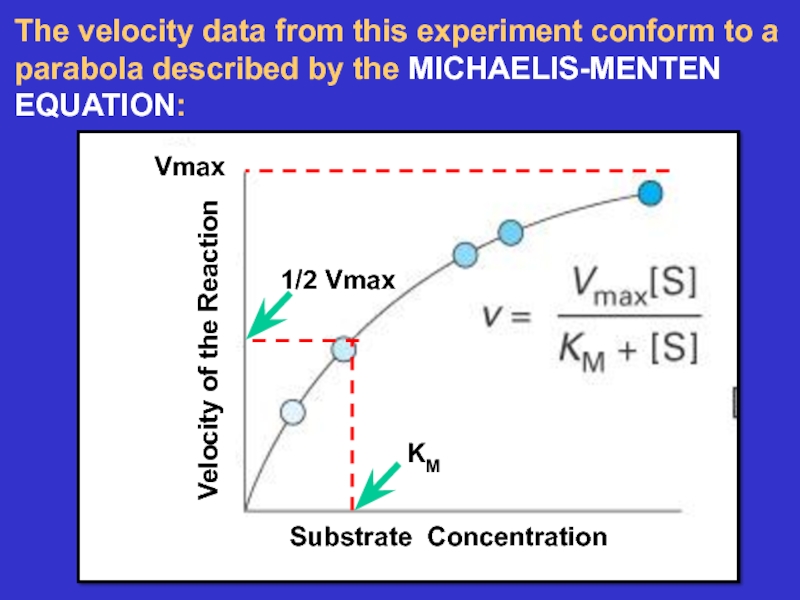
- 20. The velocity data from this experiment conform to a parabola described by the MICHAELIS-MENTEN EQUATION:
- 21. The two parameters KM and VMAX are
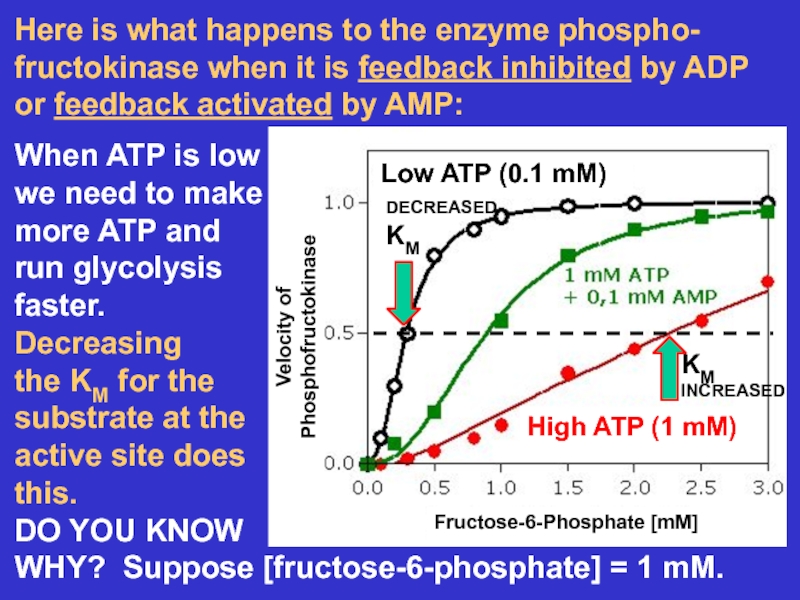
- 22. Here is what happens to the enzyme
- 23. Anaerobic glycolysis summary:It occurs in the cytoplasm
- 24. Скачать презентанцию
Слайды и текст этой презентации
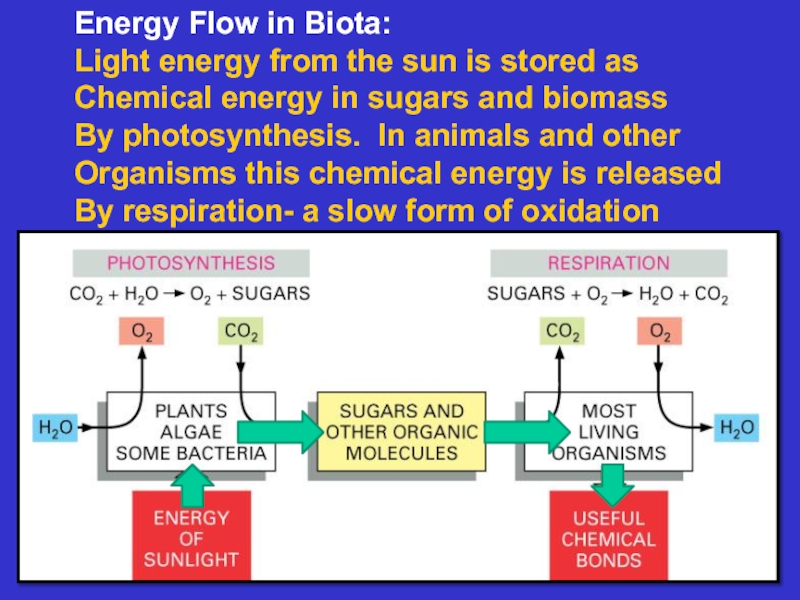
Слайд 2Energy Flow in Biota:
Light energy from the sun is stored
as
Chemical energy in sugars and biomass
By photosynthesis. In animals and
otherOrganisms this chemical energy is released
By respiration- a slow form of oxidation
Слайд 3This reaction yields energy due to breaking
of chemical bonds. The
energy is expressed
as a change in “Gibbs Free Energy:
ENERGY OF
REACTANTS – ENERGY OF PRODUCTS =CHANGE IN ENERGY = ΔG (“Gibbs Free Energy”)
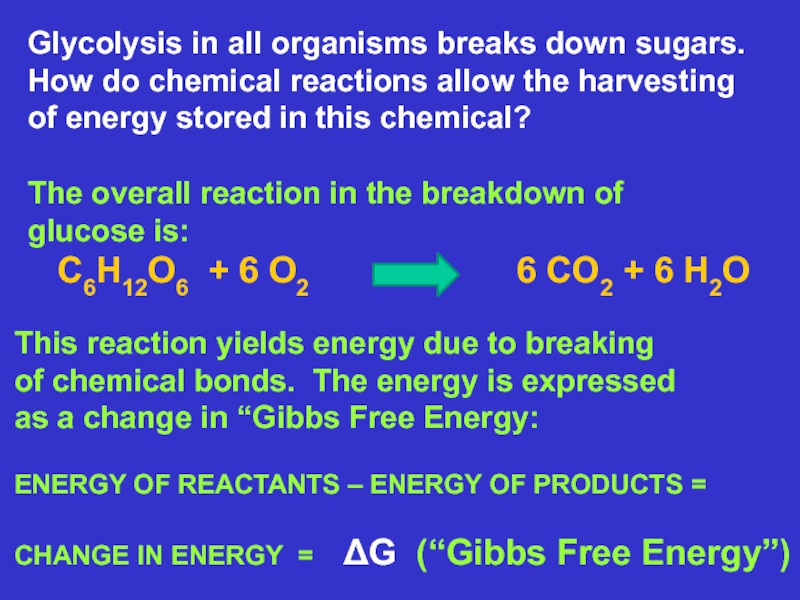
Glycolysis in all organisms breaks down sugars.
How do chemical reactions allow the harvesting
of energy stored in this chemical?
The overall reaction in the breakdown of
glucose is:
C6H12O6 + 6 O2 6 CO2 + 6 H2O
Слайд 5Now consider glycolysis!
C6H12O6 + 6 O2
6 CO2 +
6 H2OThis reaction is highly favored because
ΔG = - 686 Kilocal/Mole! (Note the minus sign!)
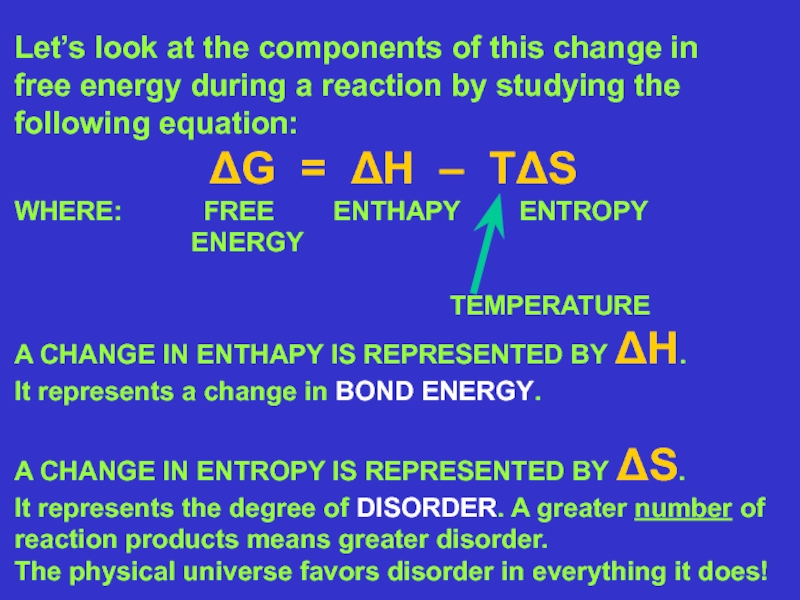
First, ΔH is negative! WHY DO YOU THINK THIS IS?
Second, ΔS is positive! WHY DO YOU THINK THIS IS?
Highly favored reactions like this are called
CATABOLIC. They release energy that can then
be used for synthesis of new macromolecules
by a cell.
Слайд 6Reactions in cells that make new macro-
molecules (proteins, DNA, RNA,
etc.) are
generally unfavorable
These reactions are called ANABOLIC or
SYNTHETIC.
They
require an input of free energy to makethem happen (go forward). Their ΔG is positive!
WHY DO YOU THINK THEIR ΔG IS POSITIVE?
Example of an anabolic reaction:
100 AMINO ACIDS PROTEIN + H2O
Слайд 7How do we transfer the energy yield from
highly favorable
CATABOLIC reactions
to unfavorable synthetic/Anabolic reactions
that make macromolecules the
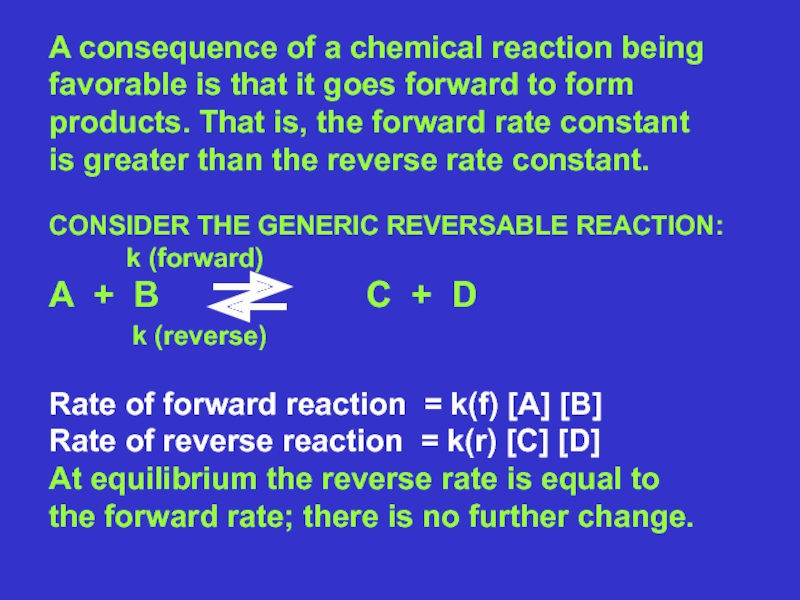
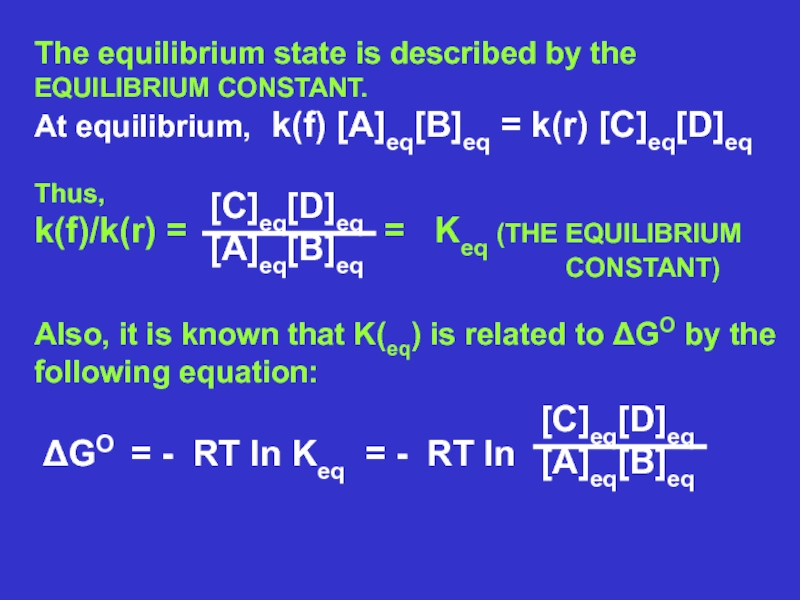
cell needs?Слайд 10A consequence of a chemical reaction being
favorable is that it
goes forward to form
products. That is, the forward rate constant
is
greater than the reverse rate constant. CONSIDER THE GENERIC REVERSABLE REACTION:
k (forward)
A + B C + D
k (reverse)
Rate of forward reaction = k(f) [A] [B]
Rate of reverse reaction = k(r) [C] [D]
At equilibrium the reverse rate is equal to
the forward rate; there is no further change.
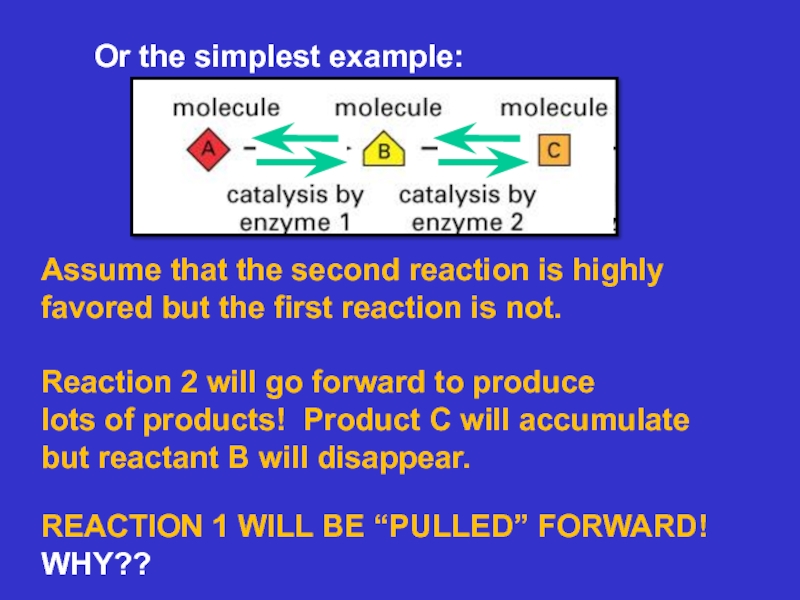
Слайд 13Or the simplest example:
Assume that the second reaction is highly
favored
but the first reaction is not.
Reaction 2 will go forward
to producelots of products! Product C will accumulate
but reactant B will disappear.
REACTION 1 WILL BE “PULLED” FORWARD!
WHY??
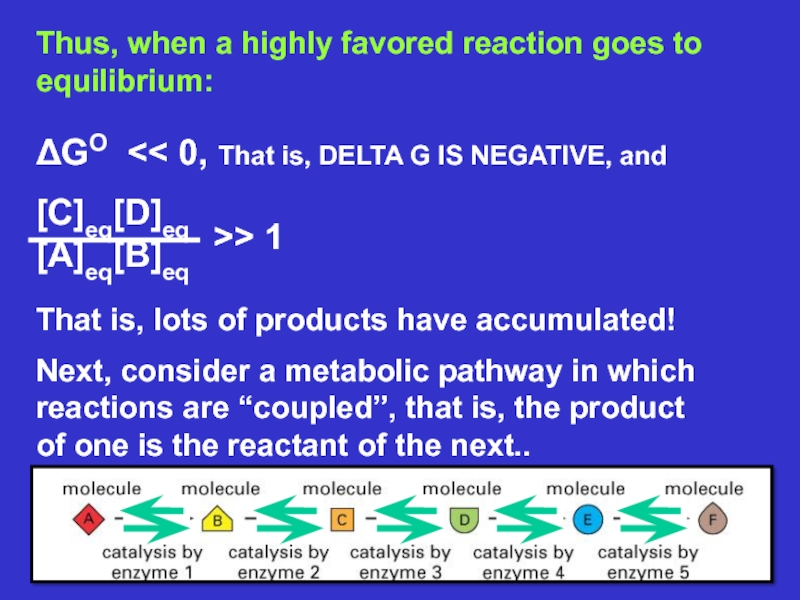
Слайд 14Glycolysis is
broken up
into ANAEROBIC
GLYCOLYSIS in
the cytoplasm
(10 STEPS) and
AEROBIC
GLYCOLYSIS in
mitochondria
(9 STEPS +
Electron
Transport)
ANAEROBIC
GLYCOLYSIS
LOOKS LIKE
THIS:
THIS IS ANAEROBIC
GLYCOLYSIS
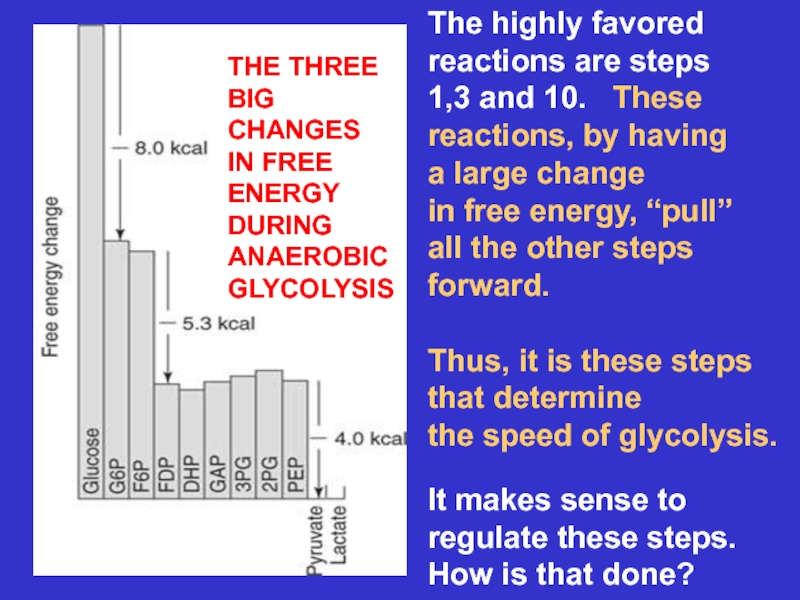
Слайд 15The highly favored
reactions are steps
1,3 and 10. These
reactions, by
having
a large change
in free energy, “pull”
all the other steps
forward.
Thus, it
is these stepsthat determine
the speed of glycolysis.
It makes sense to
regulate these steps.
How is that done?
THE THREE
BIG
CHANGES
IN FREE
ENERGY
DURING
ANAEROBIC
GLYCOLYSIS
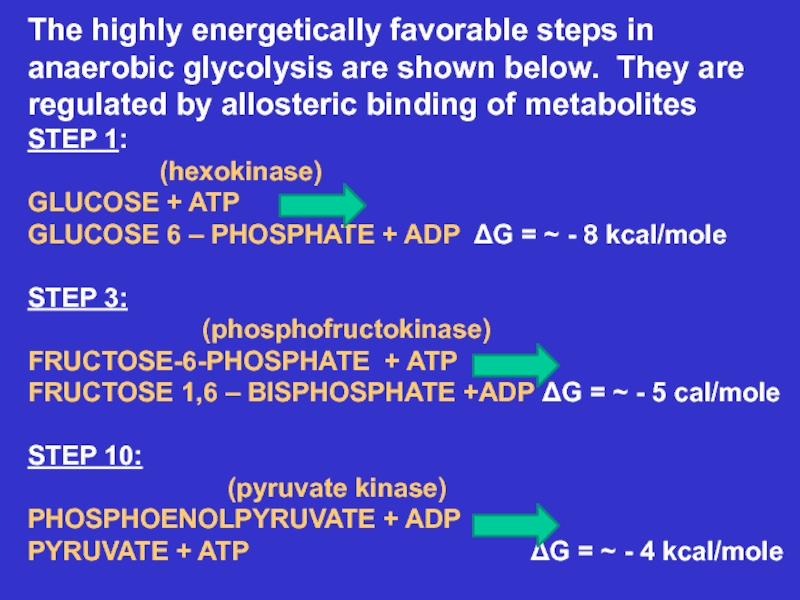
Слайд 16The highly energetically favorable steps in anaerobic glycolysis are shown
below. They are
regulated by allosteric binding of metabolites
STEP 1:
(hexokinase)GLUCOSE + ATP
GLUCOSE 6 – PHOSPHATE + ADP ΔG = ~ - 8 kcal/mole
STEP 3:
(phosphofructokinase)
FRUCTOSE-6-PHOSPHATE + ATP
FRUCTOSE 1,6 – BISPHOSPHATE +ADP ΔG = ~ - 5 cal/mole
STEP 10:
(pyruvate kinase)
PHOSPHOENOLPYRUVATE + ADP
PYRUVATE + ATP ΔG = ~ - 4 kcal/mole
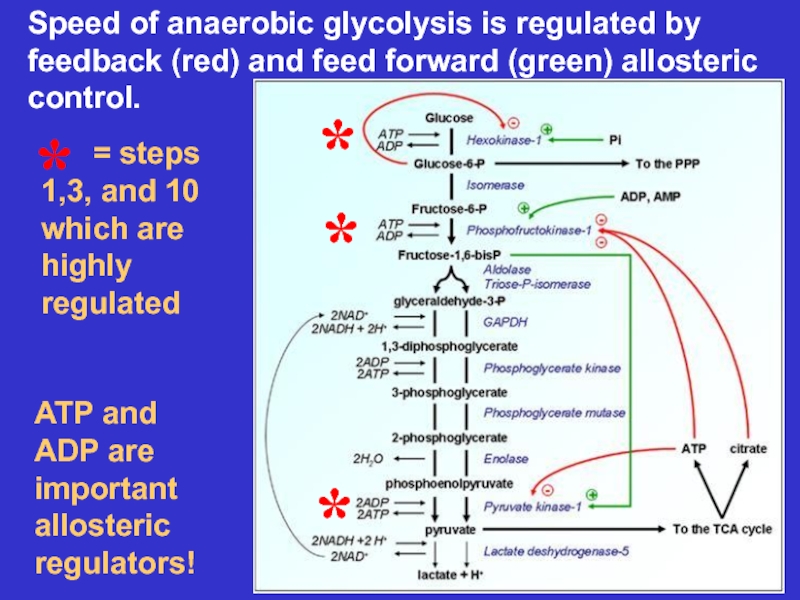
Слайд 17ATP and ADP are important allosteric regulators!
Speed of anaerobic glycolysis
is regulated by feedback (red) and feed forward (green) allosteric
control.*
*
*
*
= steps 1,3, and 10 which are highly regulated
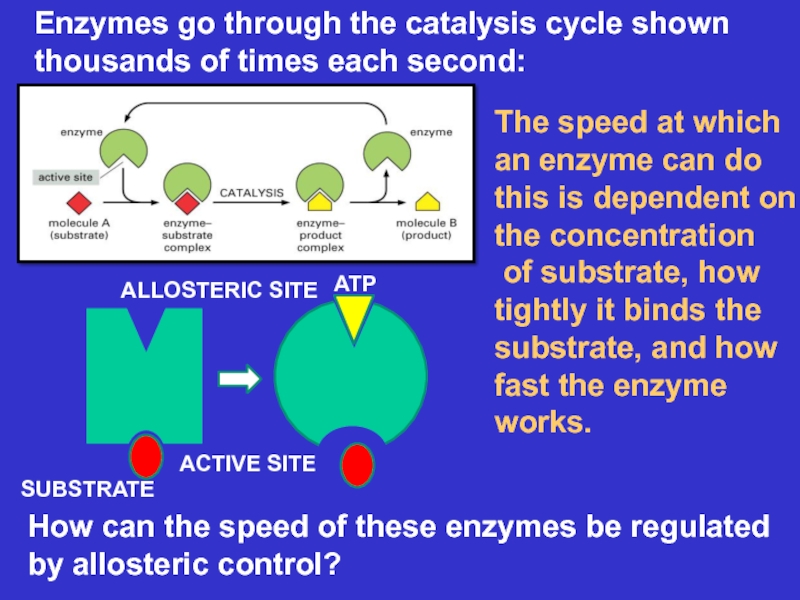
Слайд 18Enzymes go through the catalysis cycle shown thousands of times
each second:
The speed at which an enzyme can do
this is dependent on the concentrationof substrate, how tightly it binds the substrate, and how fast the enzyme works.
How can the speed of these enzymes be regulated by allosteric control?
SUBSTRATE
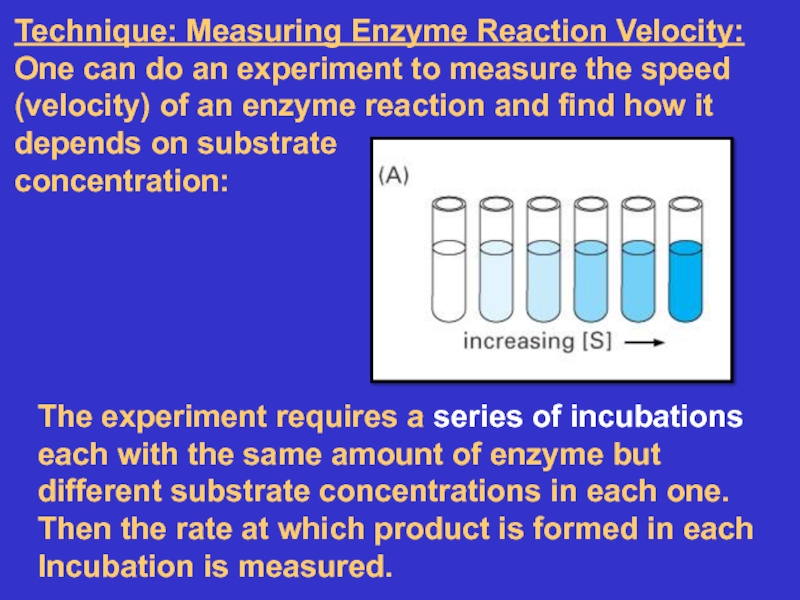
Слайд 19Technique: Measuring Enzyme Reaction Velocity: One can do an experiment
to measure the speed (velocity) of an enzyme reaction and
find how it depends on substrateconcentration:
The experiment requires a series of incubations each with the same amount of enzyme but different substrate concentrations in each one.
Then the rate at which product is formed in each
Incubation is measured.
Слайд 20The velocity data from this experiment conform to a parabola
described by the MICHAELIS-MENTEN EQUATION:
Слайд 21The two parameters KM and VMAX are characteristic
Of each enzyme-substrate
combination.
KM is the substrate concentration
required to obtain a half maximal
velocity and is related to how tightly the
enzyme binds the substrate at its active site.
VMAX is the maximal rate that the
enzyme can work at and is related
to how many enzyme proteins are present
and how fast each one works.
Allosteric regulators can change these parameters!
Слайд 22Here is what happens to the enzyme phospho-
fructokinase when it
is feedback inhibited by ADP
or feedback activated by AMP:
When ATP
is lowwe need to make
more ATP and
run glycolysis
faster.
Decreasing
the KM for the
substrate at the
active site does
this.
DO YOU KNOW
WHY? Suppose [fructose-6-phosphate] = 1 mM.
Слайд 23Anaerobic glycolysis summary:
It occurs in the cytoplasm in 10 steps
Steps
1, 3 and 10 are most favorable and
pull the pathway
forward. They are the stepsthat are most important to regulate.
3. Regulation is by ALLOSTERIC CONTROL.
Glycolysis requires the input of ATP in early
steps in order to yield more ATP at later steps.
5. Overall, the yield of ATP is very modest. Only
4 ATP molecules per molecule of glucose
processed.