Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
От спин-меченых конденсированных полиароматических соединений к
Содержание
- 1. От спин-меченых конденсированных полиароматических соединений к
- 2. • “Spin Units” have: (a)
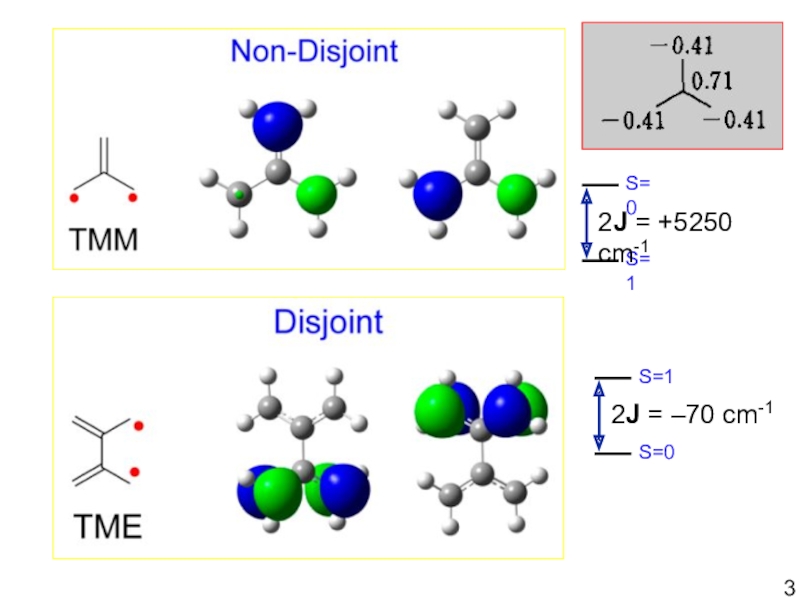
- 3. S=1S=02J = +5250 cm-1S=0S=12J = –70 cm-1
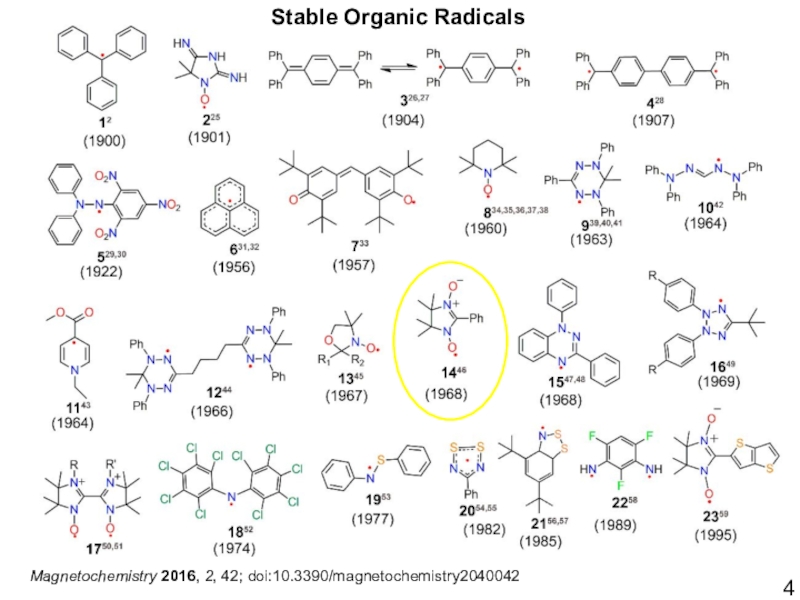
- 4. Magnetochemistry 2016, 2, 42; doi:10.3390/magnetochemistry2040042Stable Organic Radicals
- 5. Electronic structure of nitronyl nitroxide0.27–0.121Spin densityJ. Am. Chem. Soc., 116, 2019 (1994)
- 6. Approaches to Nitronyl NitroxidesAust. J. Chem., 2017, 70, 1317–1320.
- 7. C(sp2)-centered ElectrophilesDifferent type of products detected– LiH,
- 8. J. Am. Chem. Soc. 2010, 132, 15908Stable Triplet Diradical
- 9. Stable Triplet Diradical2J = +540 cm-1S=1S=0S = 1
- 10. J. Am. Chem. Soc. 2010, 132, 159082J
- 11. In solid argon15 K15 K after annealingat
- 12. Quantum chemistry 1 = 66o 1 =
- 13. Covalent bonding: Metal ComplexesJ/kB = -217 KS
- 14. H-Bonded Assembly of Nitronyl NitroxidesV. Romanov, I.
- 15. Слайд 15
- 16. 0.27–0.121Spin densityJ. Am. Chem. Soc., 116, 2019 (1994)Ferromagnet chain
- 17. Spin-related ApplicationsAnalog Electronics and SpintronicsC.E. Banks et al., Materials Today, 17, 2014, 426100 × 100 nm
- 18. Graphene nanostructuresSpin-related ApplicationsShiyong Wang et al., Nature Communications | 7:11507 | DOI: 10.1038/ncomms11507
- 19. ZGNR with atomically precise CH edgesMagnetic Properties
- 20. Possible Applications in SpintronicsElectric-field-induced half-metallicitySon et al.,
- 21. Synthetic GrapheneChemical vapour deposition (CVD)Toshiaki Enoki, Phys.
- 22. Applicable Graphene MaterialsRegular shapeControlled azimuthal orientationMagnetically precise
- 23. P. Ruffieux et al., 10.1038/nature17151On Surface Syntheses of Graphene Materials
- 24. C. Moreno et al., Science 360, 199
- 25. K. Müllen and A. Narita et al.,
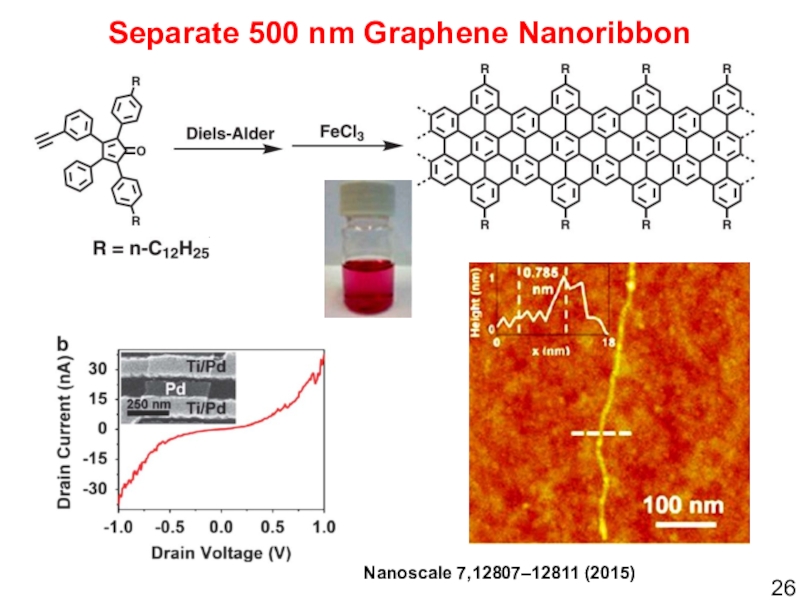
- 26. Nanoscale 7,12807–12811 (2015)Separate 500 nm Graphene Nanoribbon
- 27. Graphene zig-zag edges are very sensitive due
- 28. Graphene zig-zag edges are very sensitive due
- 29. Graphene zig-zag edges are very sensitive due
- 30. M. Slota et al., Nature, 2018, 557, 691-695.Nadezhda TroshkovaYurii Ten
- 31. MALDI-TOF MS spectra of low molecular weight
- 32. M. Slota et al., Nature, 2018, 557,
- 33. M. Slota et al., Nature, 2018, 557, 691-695.
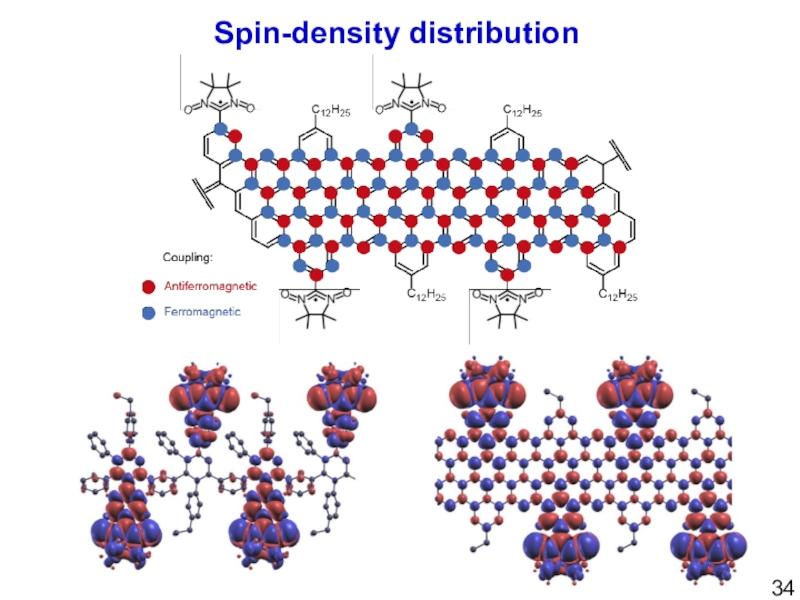
- 34. Spin-density distribution
- 35. NIT-polyphenyleneMw = 161 kg mol–1, Mn =
- 36. J1= –25±5 MHz, J2= 12±3 MHzM. Slota et al., Nature, 2018, 557, 691-695.
- 37. Exchange Interactions in NIT-GNRExperiment:J12= −8.3∙10–4 cm–1J13= 4.0∙10–4
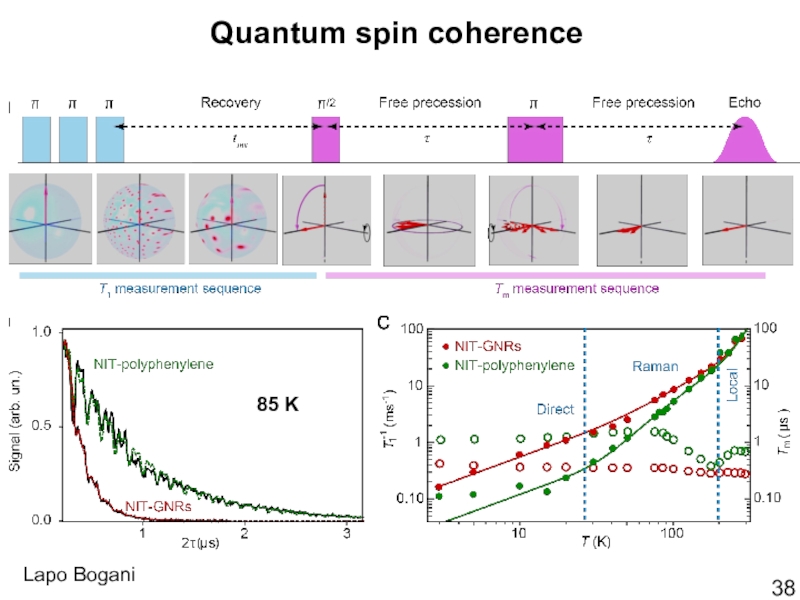
- 38. Quantum spin coherence85 KLapo Bogani
- 39. Coupling between localized spins and the edge
- 40. Coupling between localized spins and the edge
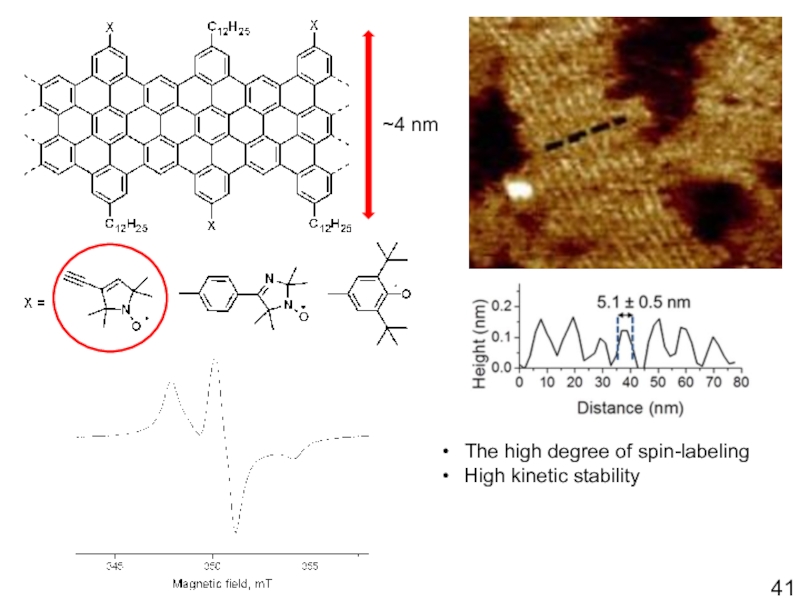
- 41. The high degree of spin-labelingHigh kinetic stability~4 nm
- 42. Syntheses of atomically and magnetically precise graphene
- 43. J = +370 KJ = 12±3 MHzPure organic magnets
- 44. 0.27–0.121Spin densityJ. Am. Chem. Soc., 116, 2019 (1994)Metal-radical graphene-like magnets
- 45. Finite polynuclear heterospyn systems D. Luneau "Molecular
- 46. 2D MnII-radical frameworks Honeycomb-like structure with intercalated
- 47. Representation of the various investigations on MnII-nitronyl nitroxide systems
- 48. Crystals, 2018, 8, 334Crystals, 2019, 9, 219
- 49. {[Mn2(NIT(Me,Me)Im)3ClO4]}nUnpublished data
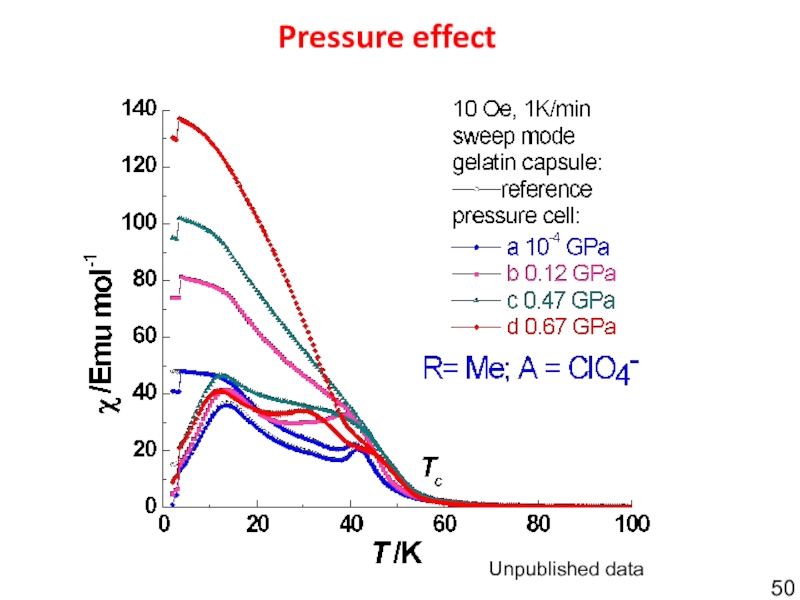
- 50. Pressure effectUnpublished data
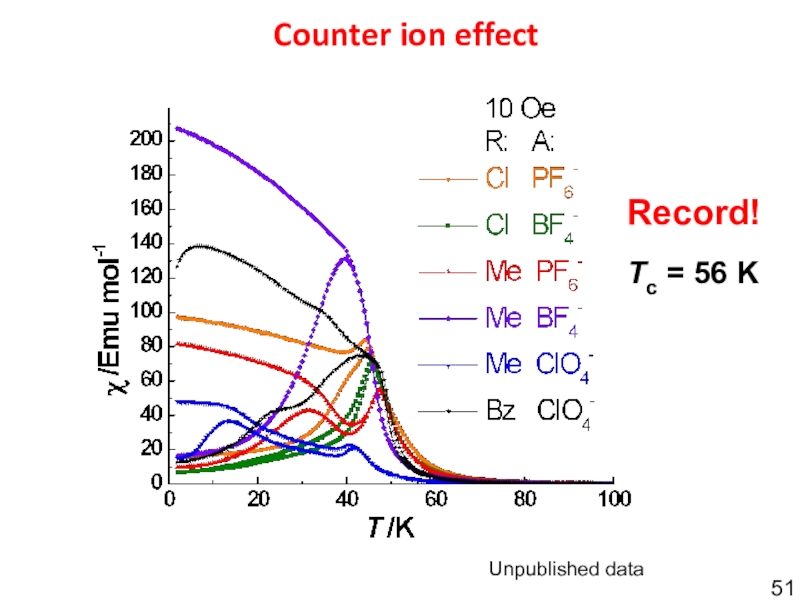
- 51. Unpublished dataCounter ion effectTc = 56 KRecord!
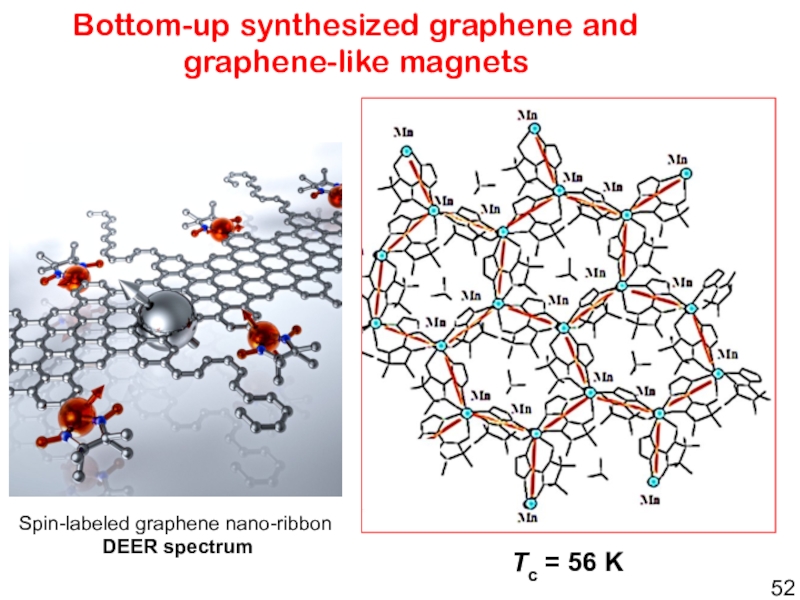
- 52. Bottom-up synthesized graphene and graphene-like magnets Tc = 56 KSpin-labeled graphene nano-ribbonDEER spectrum
- 53. Tretyakov Research GroupFunctional Organic and Hybrid Materials2020
- 54. Financial support:Deutscher Akademischer Austauschdienst The Russian Science
- 55. Скачать презентанцию
Слайды и текст этой презентации
Слайд 1От спин-меченых конденсированных полиароматических соединений к магнитно-активным графеновым наноструктурам
Е.В. Третьяков
Цикл
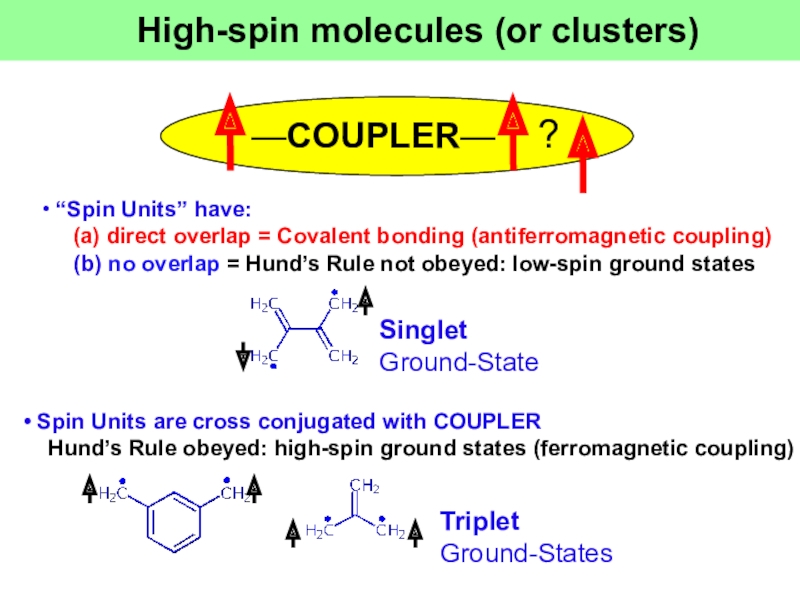
Слайд 2• “Spin Units” have:
(a) direct overlap =
Covalent bonding (antiferromagnetic coupling)
—COUPLER—
?
High-spin molecules (or clusters)
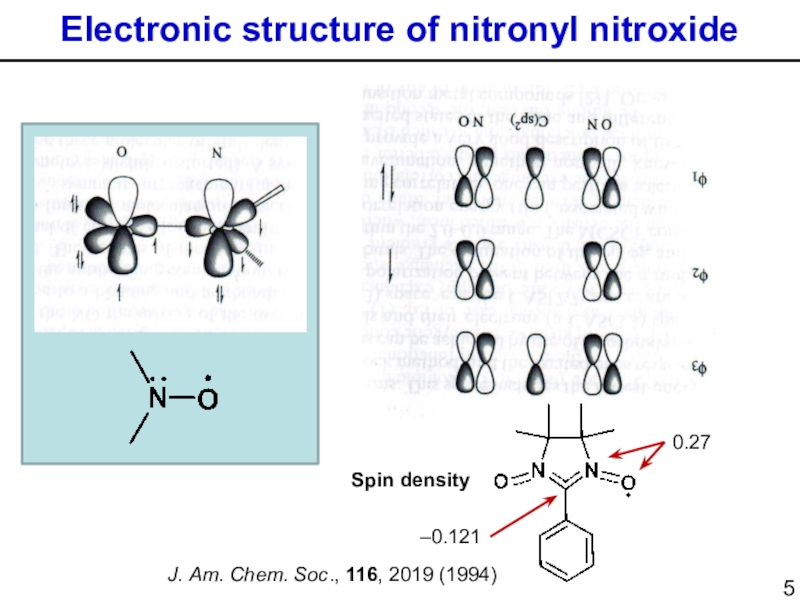
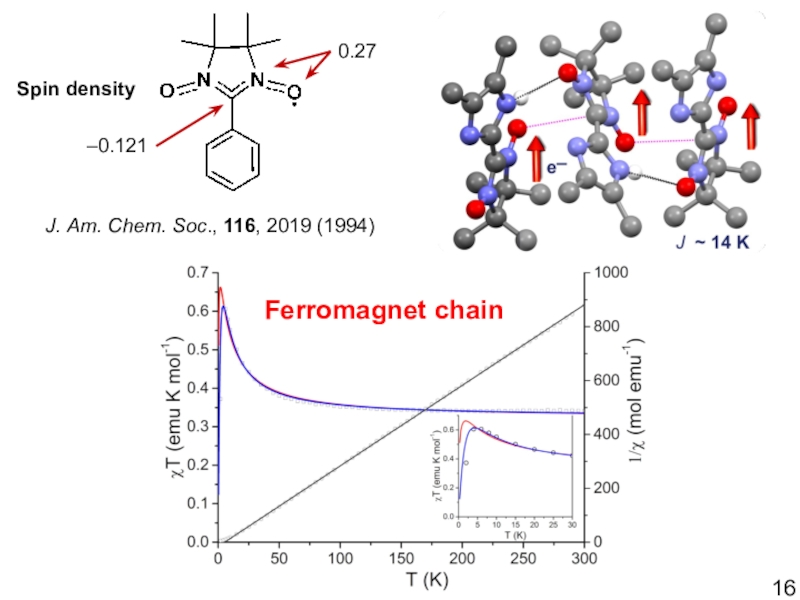
Слайд 5Electronic structure of nitronyl nitroxide
0.27
–0.121
Spin density
J. Am. Chem. Soc., 116,
2019 (1994)
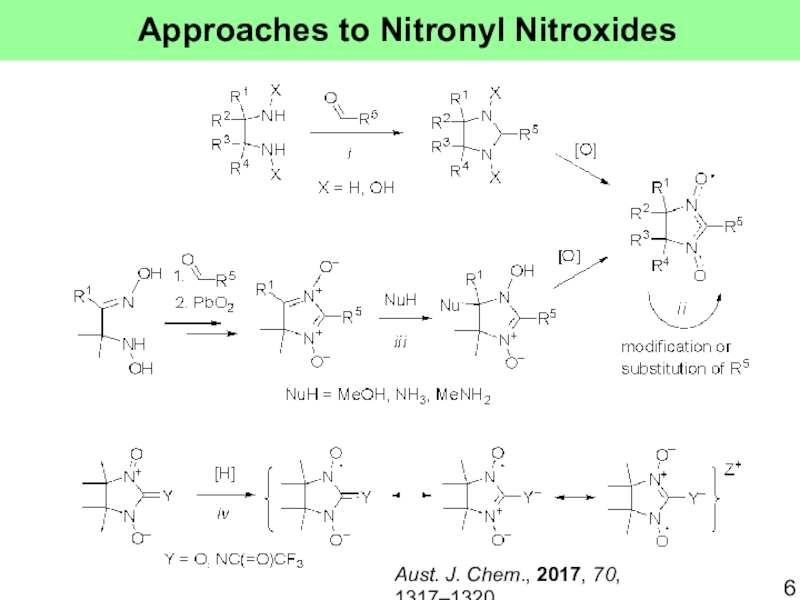
Слайд 7C(sp2)-centered Electrophiles
Different type of products detected
– LiH, Oxidative way
– LiOH,
Eliminative way
Transformation of Electrophile
J. Org. Chem. 74, 2870 (2009).
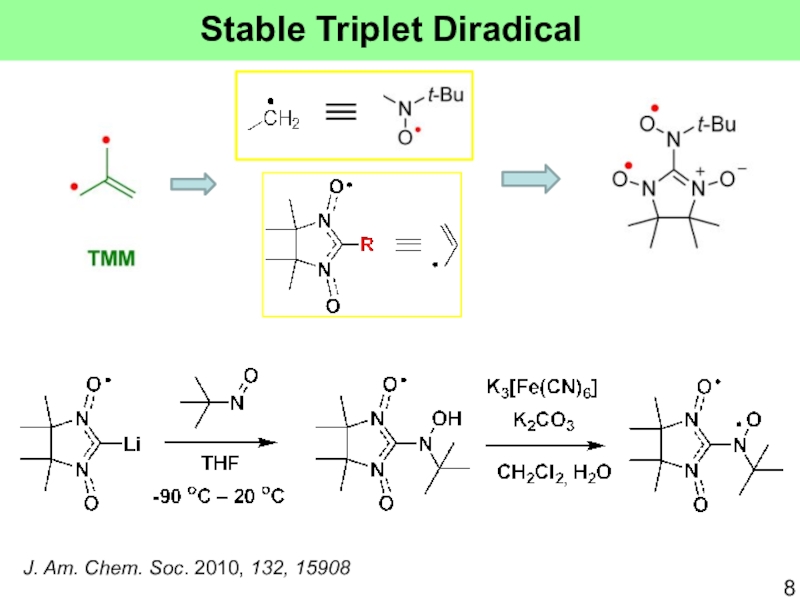
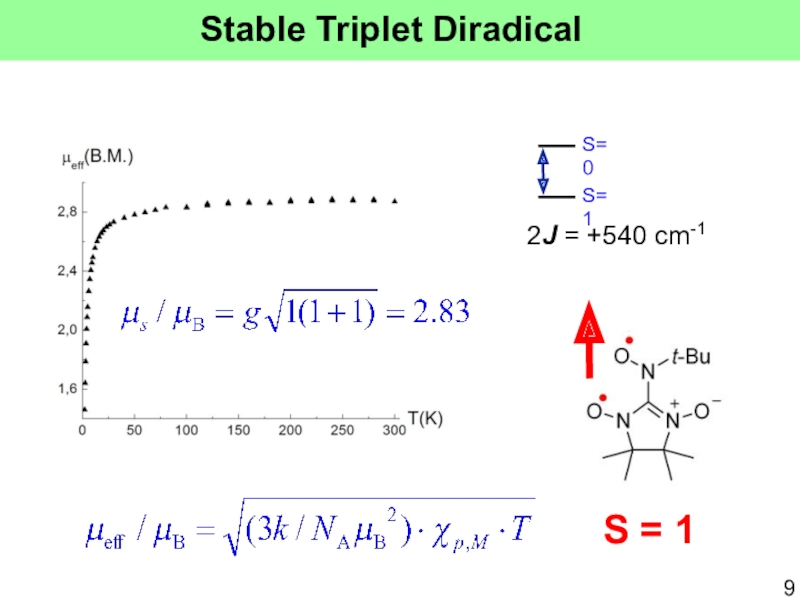
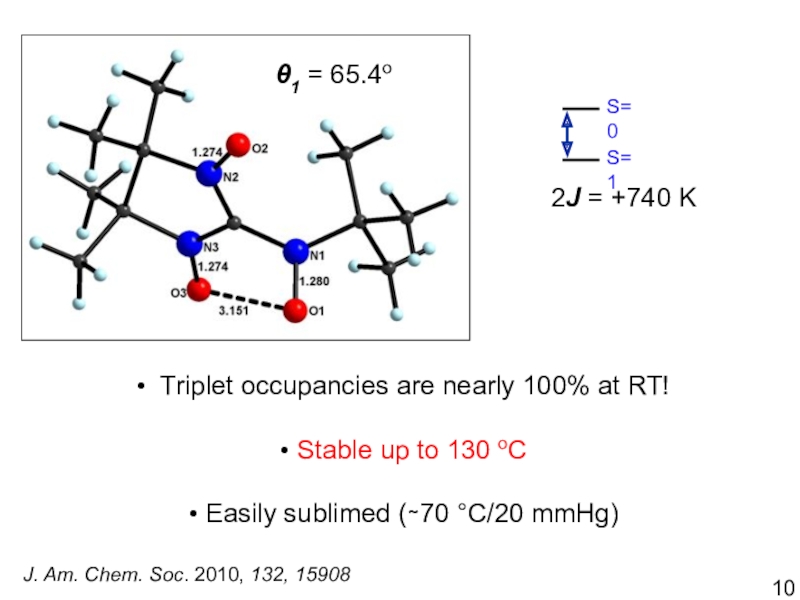
Слайд 10J. Am. Chem. Soc. 2010, 132, 15908
2J = +740 K
S=1
S=0
Triplet occupancies are nearly 100% at RT!
Stable up to
130 oCEasily sublimed (∼70 °C/20 mmHg)
1 = 65.4o
Слайд 11In solid argon
15 K
15 K after annealing
at 28 K
D =
0.0248 cm-1
E = 0.0025 cm-1
Ar/DR 103
J. Phys.
Chem. A 2013, 117, 8065Слайд 12Quantum chemistry 1 = 66o 1 = 16.5o
Stability of Conformations
The
pseudo-eclipsed (PM, MP) conformation is 1.0 kcal/mol more stable
Crystallography 1
= 65.4o and 1 = 13.9o D = -0.030 cm-1
E/D = 0.11
Rigid Diradical
J. Phys. Chem. A 2013, 117, 8065
Слайд 13Covalent bonding: Metal Complexes
J/kB = -217 K
S = 5/2
S =
1
Inorg. Chem. 2014, 53, 802−809.
1 = 29o
1 = 65.4o
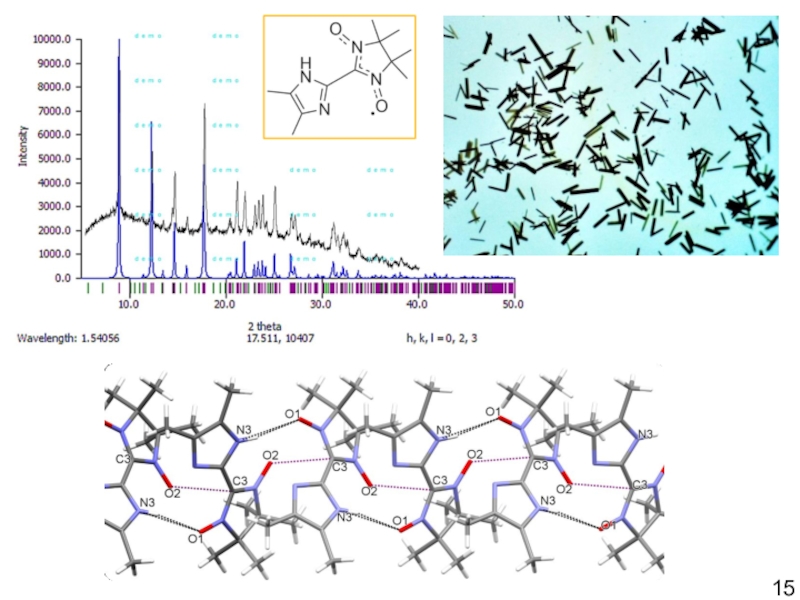
Слайд 14H-Bonded Assembly of Nitronyl Nitroxides
V. Romanov, I. Bagryanskaya, N. Gritsan,
D. Gorbunov, Yu. Vlasenko, M. Yusubov, E. Zaytseva, D. Luneau,
E. Tretyakov. Crystals 2019, 9, 219; doi:10.3390/cryst9040219Слайд 17Spin-related Applications
Analog Electronics and Spintronics
C.E. Banks et al., Materials Today,
17, 2014, 426
100 × 100 nm
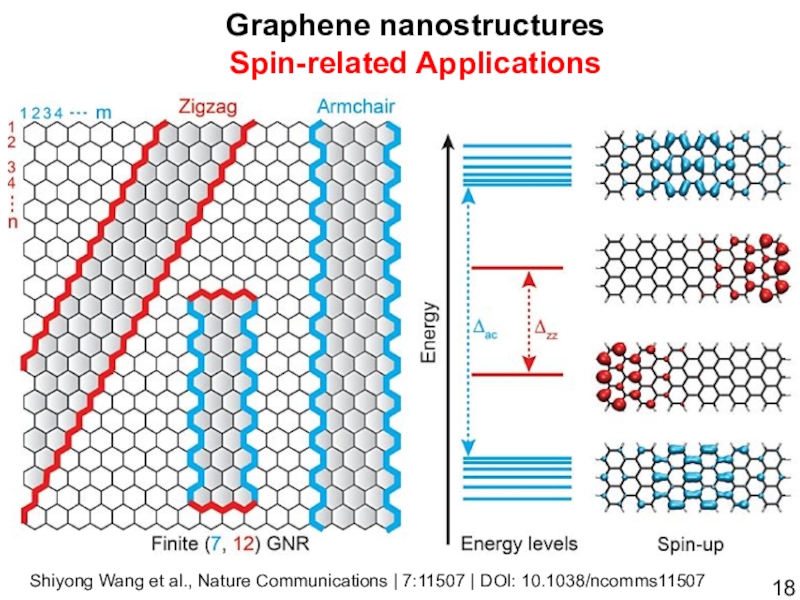
Слайд 18Graphene nanostructures
Spin-related Applications
Shiyong Wang et al., Nature Communications | 7:11507
| DOI: 10.1038/ncomms11507
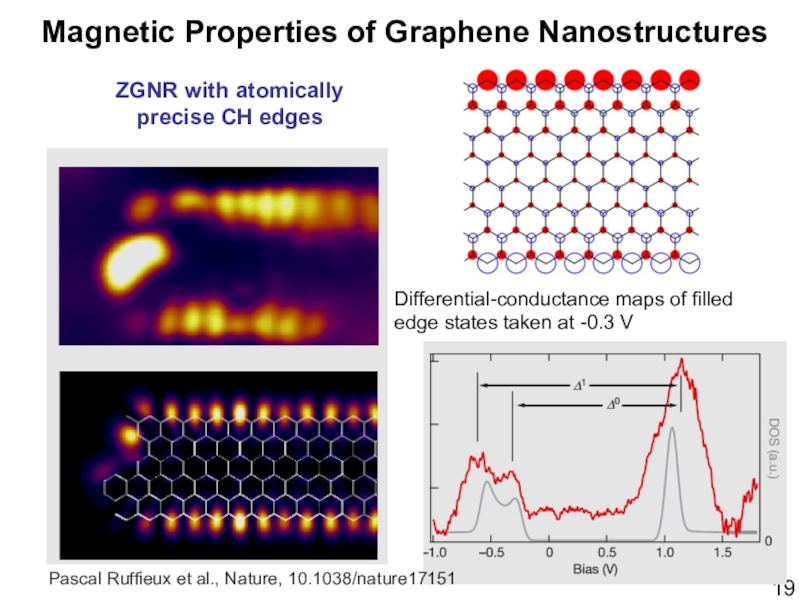
Слайд 19ZGNR with atomically precise CH edges
Magnetic Properties of Graphene Nanostructures
Pascal
Ruffieux et al., Nature, 10.1038/nature17151
Differential-conductance maps of filled
edge states taken
at -0.3 V
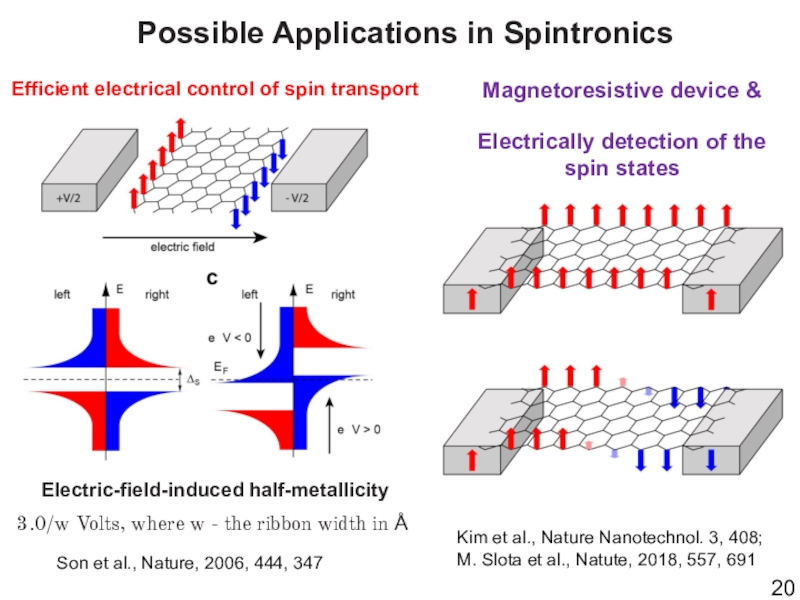
Слайд 20Possible Applications in Spintronics
Electric-field-induced half-metallicity
Son et al., Nature, 2006, 444,
347
3.0/w Volts, where w - the ribbon width in Å
Efficient
electrical control of spin transportMagnetoresistive device &
Electrically detection of the spin states
Kim et al., Nature Nanotechnol. 3, 408;
M. Slota et al., Natute, 2018, 557, 691
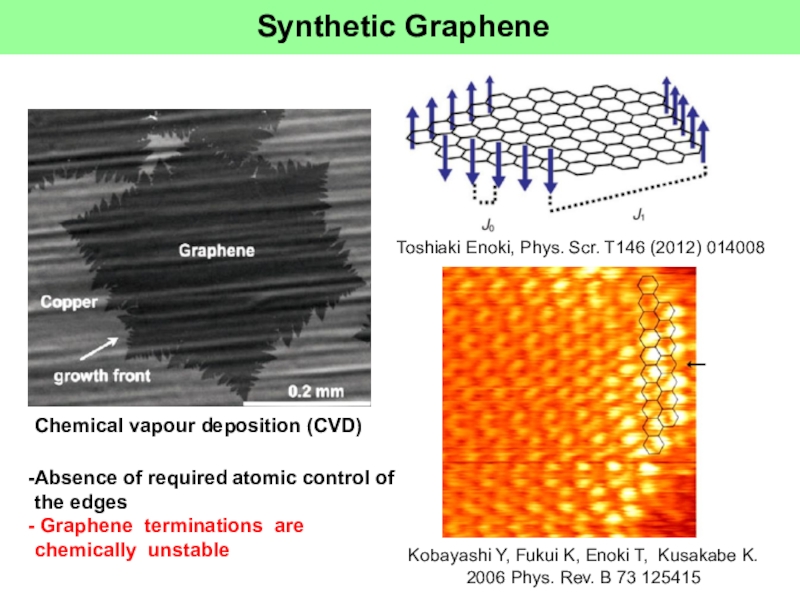
Слайд 21Synthetic Graphene
Chemical vapour deposition (CVD)
Toshiaki Enoki, Phys. Scr. T146 (2012)
014008
Kobayashi Y, Fukui K, Enoki T, Kusakabe K. 2006 Phys.
Rev. B 73 125415Absence of required atomic control of the edges
Graphene terminations are chemically unstable
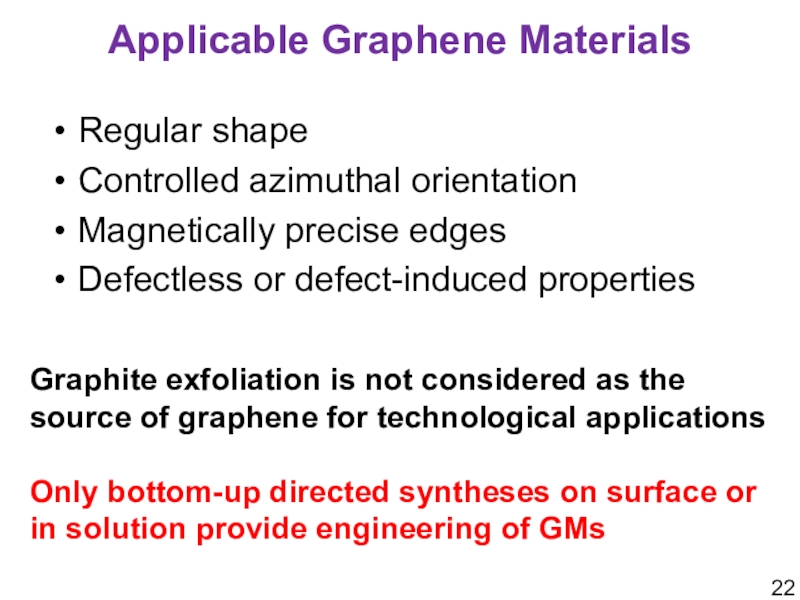
Слайд 22Applicable Graphene Materials
Regular shape
Controlled azimuthal orientation
Magnetically precise edges
Defectless or defect-induced
properties
Graphite exfoliation is not considered as the source of graphene
for technological applicationsOnly bottom-up directed syntheses on surface or in solution provide engineering of GMs
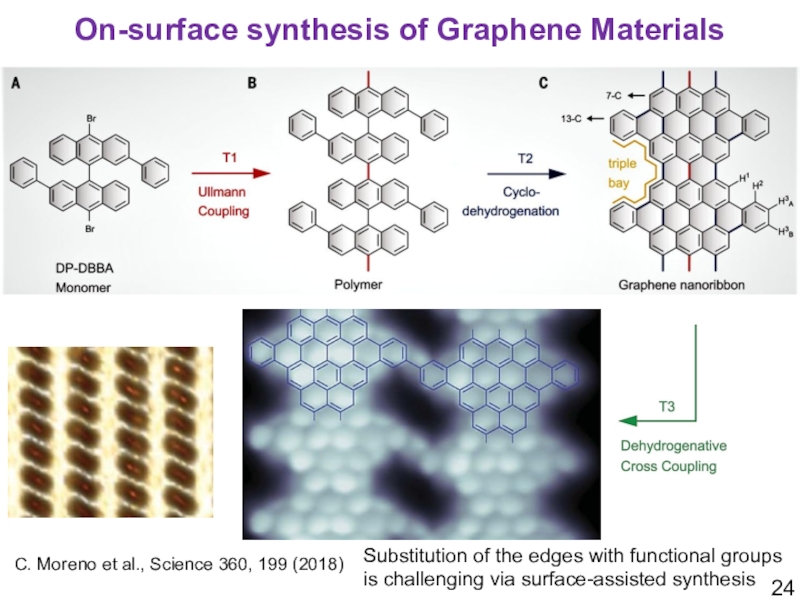
Слайд 24C. Moreno et al., Science 360, 199 (2018)
On-surface synthesis of
Graphene Materials
Substitution of the edges with functional groups is challenging
via surface-assisted synthesisСлайд 25K. Müllen and A. Narita et al., JACS, 2018, DOI:
10.1021/jacs.8b02209
Solution-mediated Synthesis of Graphene Nanostructures
up to ~ 60 nm in
length250-260 oC
Слайд 27Graphene zig-zag edges are very sensitive due to radicaloid character
Attempts
of solution-mediated synthesis of zig-zag nano-ribbons failed
Not-stable zig-zag GNR
Слайд 28Graphene zig-zag edges are very sensitive due to radicaloid character
Attempts
of solution-mediated synthesis of zig-zag nano-ribbons failed
Not-stable zig-zag GNR
Stable Nitroxides
K.
Okada, et al,. Chem. Lett. 2014, 43, 678.M. Haraguchi, et al. Chem. Asian J., 2017, 12, 2929
E. Tretyakov, et al., ChemistryOpen, 2017, 6, 642
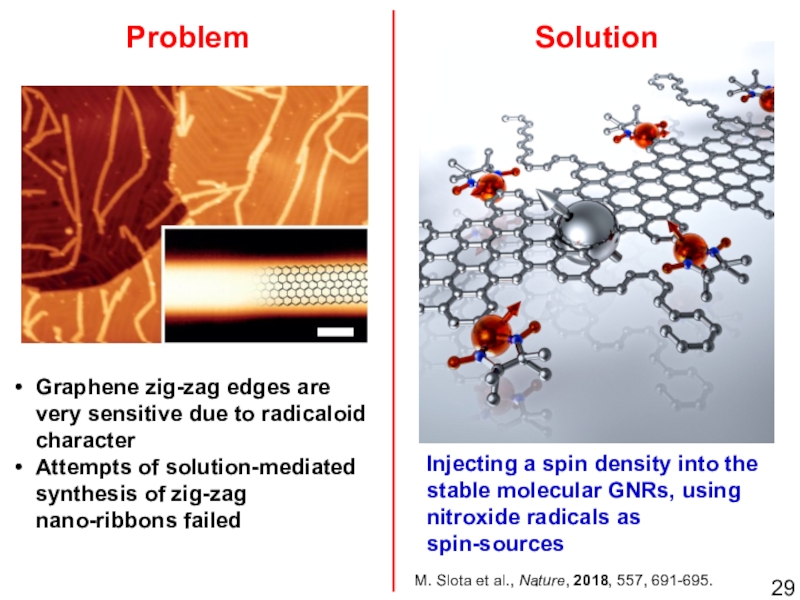
Слайд 29Graphene zig-zag edges are very sensitive due to radicaloid character
Attempts
of solution-mediated synthesis of zig-zag nano-ribbons failed
Problem
Solution
Injecting a spin density
into the stable molecular GNRs, using nitroxide radicals as spin-sourcesM. Slota et al., Nature, 2018, 557, 691-695.
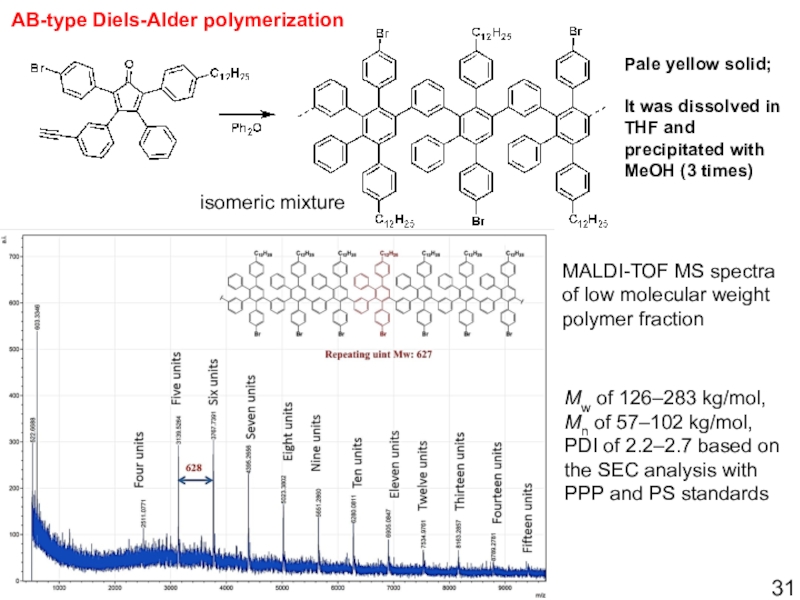
Слайд 31MALDI-TOF MS spectra of low molecular weight polymer fraction
Mw of
126–283 kg/mol, Mn of 57–102 kg/mol, PDI of 2.2–2.7 based
on the SEC analysis with PPP and PS standardsAB-type Diels-Alder polymerization
Pale yellow solid;
It was dissolved in THF and precipitated with MeOH (3 times)
isomeric mixture
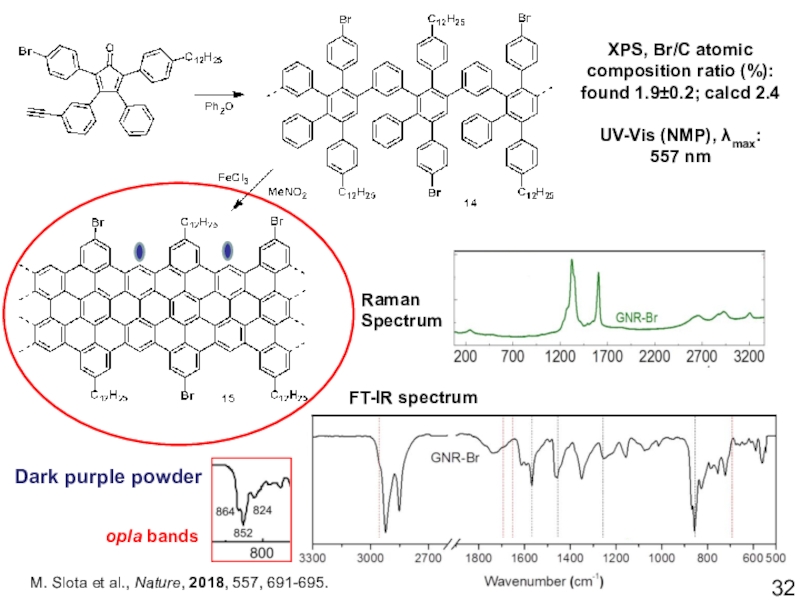
Слайд 32M. Slota et al., Nature, 2018, 557, 691-695.
Raman Spectrum
FT-IR spectrum
Dark
purple powder
XPS, Br/C atomic composition ratio (%):
found 1.9±0.2; calcd
2.4UV-Vis (NMP), λmax:
557 nm
opla bands
Слайд 35NIT-polyphenylene
Mw = 161 kg mol–1, Mn = 56 kg mol–1,
and PDI = 2.9 based on SEC analysis against PPP
standardsNIT-GNR
100 nm average length
NIT-polyphenylene
NIT-GNR
M. Slota et al., Nature, 2018, 557, 691-695.
Слайд 37Exchange Interactions in NIT-GNR
Experiment:
J12= −8.3∙10–4 cm–1
J13= 4.0∙10–4 cm–1
The degrees of
spin labeling of NIT-GNR is near 1.3%.
V. Morozov, E. Tretyakov.
J. Mol. Model., 2019, 25, 58. D. Stass, E. Tretyakov. Magnetochemistry, 2019, 5(2), 32.
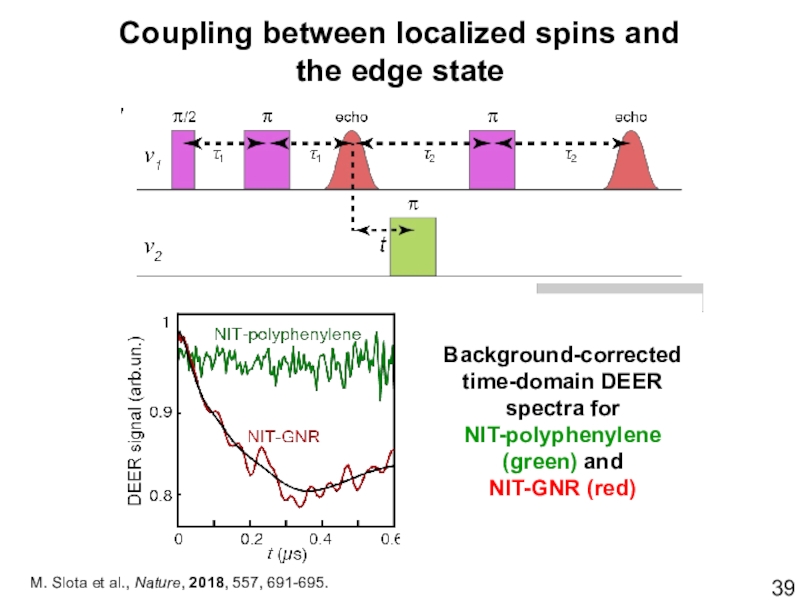
Слайд 39Coupling between localized spins and
the edge state
M. Slota et al.,
Nature, 2018, 557, 691-695.
Background-corrected time-domain DEER spectra for
NIT-polyphenylene (green) and
NIT-GNR
(red)Слайд 40Coupling between localized spins and
the edge state
M. Slota et al.,
Nature, 2018, 557, 691-695.
FFT of the DEER signal yields a
radical–edge spin interaction of 1.5 MHz. The edge–radical spin inversion time ~330 ns is considerably shorter than Tm, enabling coherent inversion operations using graphene edge states and localized spins.Слайд 42Syntheses of atomically and magnetically precise graphene nanoribbons
Graphene Boom,
Quo Vadis?
Characterization is challenging
Aggregation-related problems
Mechanical manipulation is a special task
Electrical
contactTake home message:
Magnetically- and spin-state-responding organic (semi)conductors
Слайд 440.27
–0.121
Spin density
J. Am. Chem. Soc., 116, 2019 (1994)
Metal-radical graphene-like magnets
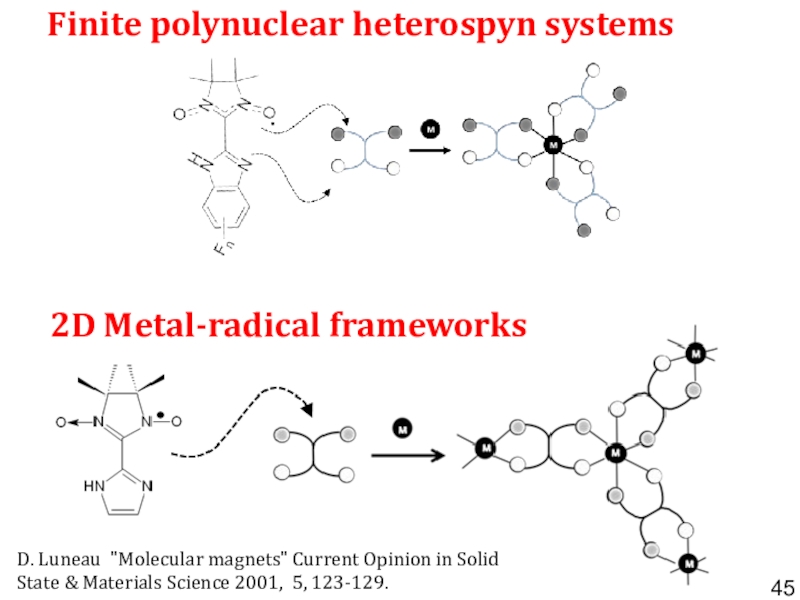
Слайд 45Finite polynuclear heterospyn systems
D. Luneau "Molecular magnets" Current Opinion
in Solid State & Materials Science 2001, 5, 123-129.
2D Metal-radical
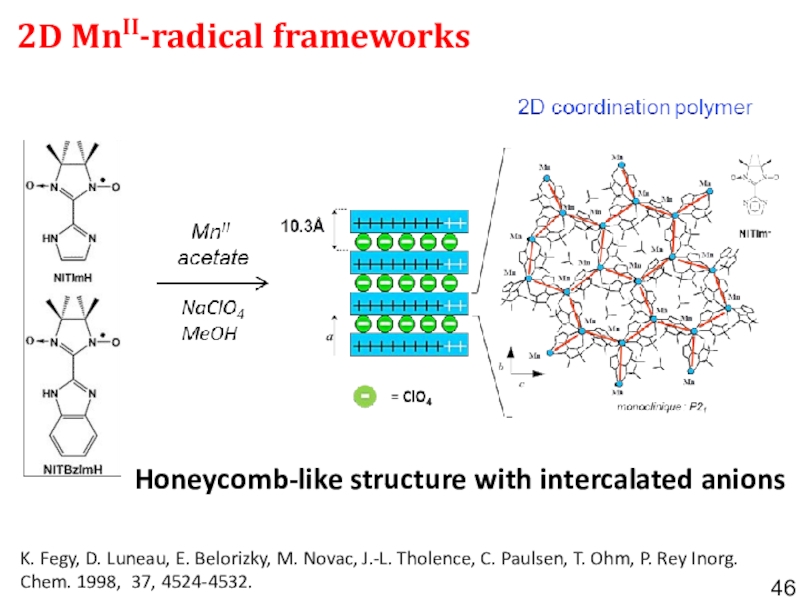
frameworks Слайд 462D MnII-radical frameworks
Honeycomb-like structure with intercalated anions
K. Fegy,
D. Luneau, E. Belorizky, M. Novac, J.-L. Tholence, C. Paulsen,
T. Ohm, P. Rey Inorg. Chem. 1998, 37, 4524-4532.Слайд 52Bottom-up synthesized graphene and graphene-like magnets
Tc = 56 K
Spin-labeled
graphene nano-ribbon
DEER spectrum
Слайд 54Financial support:
Deutscher Akademischer Austauschdienst
The Russian Science Foundation
the Ministry of
Science and Higher Education
(RFMEFI61619X0116)
Max Planck Institute for Polymer
ResearchKlaus Müllen
Martin Baumgarten
Akimitsu Narita
Acknowledgments
University of Oxford
Lapo Bogani
Michael Slota
William K. Myers
Lancaster University
Hatef Sadeghi
Colin J. Lambert
Novosibirsk Institute of
Organic Chemistry
Elena Bagryanskaya
Elena Zaytseva
University of Manchester
Ashok Keerthi
Universit´e Claude Bernard Lyon-1
Dominique Luneau





















![От спин-меченых конденсированных полиароматических соединений к {[Mn2(NIT(Me,Me)Im)3ClO4]}nUnpublished data {[Mn2(NIT(Me,Me)Im)3ClO4]}nUnpublished data](/img/tmb/7/621352/aa003b5f9a2e3d81b3fcff0a41839f0a-800x.jpg)