Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
PROTEINS
Содержание
- 1. PROTEINS
- 2. Early nutritional scientists such as the German Carl
- 3. Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ᵻnz/) are large biomolecules, or macromolecules, consisting of one
- 4. Resonance structures of the peptide bond that links individual amino
- 5. It has been estimated that average-sized bacteria contain about
- 6. Primary structure: the amino acid sequence. A protein
- 7. Cellular functions Proteins are the chief actors
- 8. Constituent amino-acids can be analyzed to predict
- 9. Скачать презентанцию
Early nutritional scientists such as the German Carl von Voit believed that protein was the most important nutrient for maintaining the structure of the body, because it was generally believed that "flesh makes
Слайды и текст этой презентации
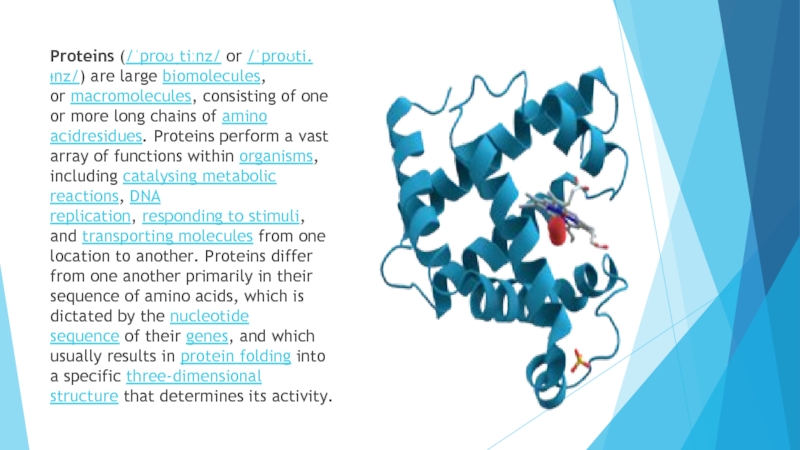
Слайд 3Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ᵻnz/) are large biomolecules, or macromolecules, consisting of one or more long
chains of amino acidresidues. Proteins perform a vast array of functions
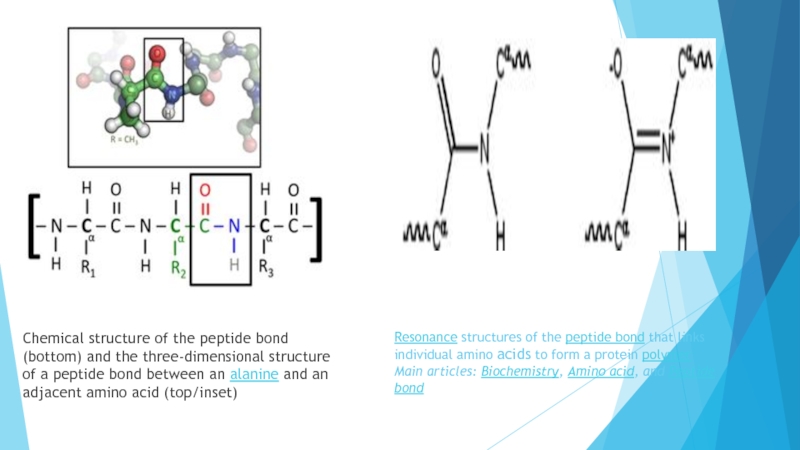
within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.Слайд 4Resonance structures of the peptide bond that links individual amino acids to form
a protein polymer
Main articles: Biochemistry, Amino acid, and Peptide bond
Chemical structure of the peptide
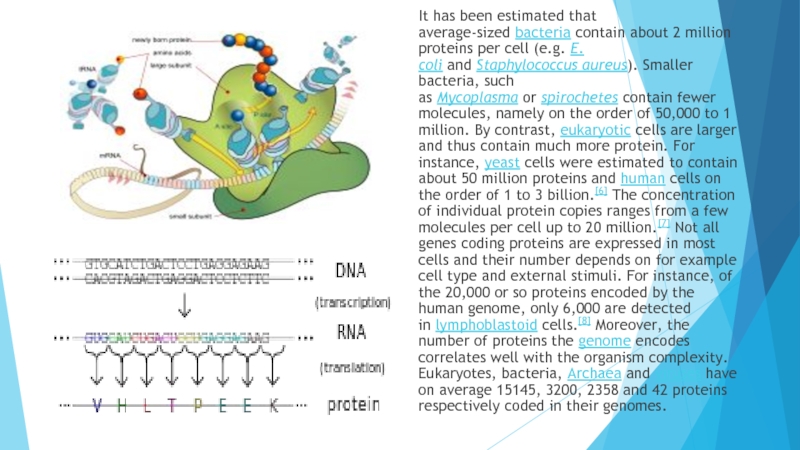
bond (bottom) and the three-dimensional structure of a peptide bond between an alanine and an adjacent amino acid (top/inset)Слайд 5It has been estimated that average-sized bacteria contain about 2 million proteins
per cell (e.g. E. coli and Staphylococcus aureus). Smaller bacteria, such as Mycoplasma or spirochetes contain fewer
molecules, namely on the order of 50,000 to 1 million. By contrast, eukaryotic cells are larger and thus contain much more protein. For instance, yeast cells were estimated to contain about 50 million proteins and human cells on the order of 1 to 3 billion.[6] The concentration of individual protein copies ranges from a few molecules per cell up to 20 million.[7] Not all genes coding proteins are expressed in most cells and their number depends on for example cell type and external stimuli. For instance, of the 20,000 or so proteins encoded by the human genome, only 6,000 are detected in lymphoblastoid cells.[8] Moreover, the number of proteins the genome encodes correlates well with the organism complexity. Eukaryotes, bacteria, Archaea and viruses have on average 15145, 3200, 2358 and 42 proteins respectively coded in their genomes.Слайд 6Primary structure: the amino acid sequence. A protein is a polyamide.
Secondary structure:
regularly repeating local structures stabilized by hydrogen bonds. The most common
examples are the α-helix, β-sheet and turns. Because secondary structures are local, many regions of different secondary structure can be present in the same protein molecule.Tertiary structure: the overall shape of a single protein molecule; the spatial relationship of the secondary structures to one another. Tertiary structure is generally stabilized by nonlocal interactions, most commonly the formation of a hydrophobic core, but also through salt bridges, hydrogen bonds, disulfide bonds, and even posttranslational modifications. The term "tertiary structure" is often used as synonymous with the term fold. The tertiary structure is what controls the basic function of the protein.
Quaternary structure: the structure formed by several protein molecules (polypeptide chains), usually called protein subunits in this context, which function as a single protein complex.
Слайд 7Cellular functions
Proteins are the chief actors within the cell, said
to be carrying out the duties specified by the information
encoded in genes.[5] With the exception of certain types of RNA, most other biological molecules are relatively inert elements upon which proteins act. Proteins make up half the dry weight of an Escherichia coli cell, whereas other macromolecules such as DNA and RNA make up only 3% and 20%, respectively.[27] The set of proteins expressed in a particular cell or cell type is known as its proteome.The enzyme hexokinase is shown as a conventional ball-and-stick molecular model. To scale in the top right-hand corner are two of its substrates, ATP and glucose.