Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
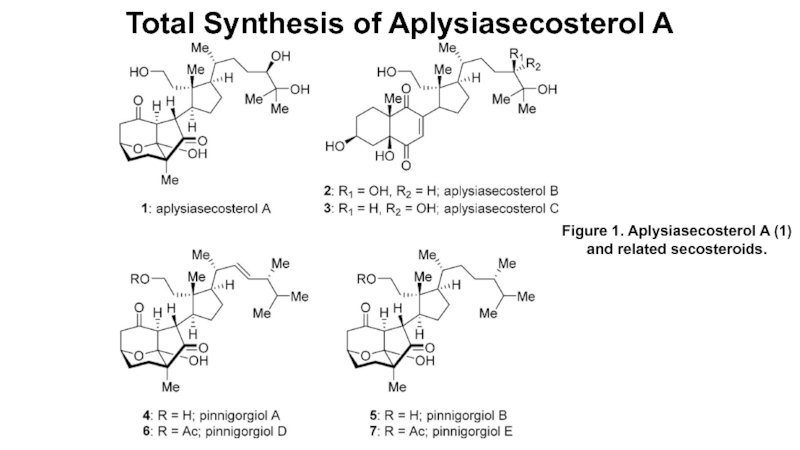
Total Synthesis of Aplysiasecosterol A
Содержание
- 1. Total Synthesis of Aplysiasecosterol A
- 2. Figure 2. Retrosynthetic analysis of aplysiasecosterol A
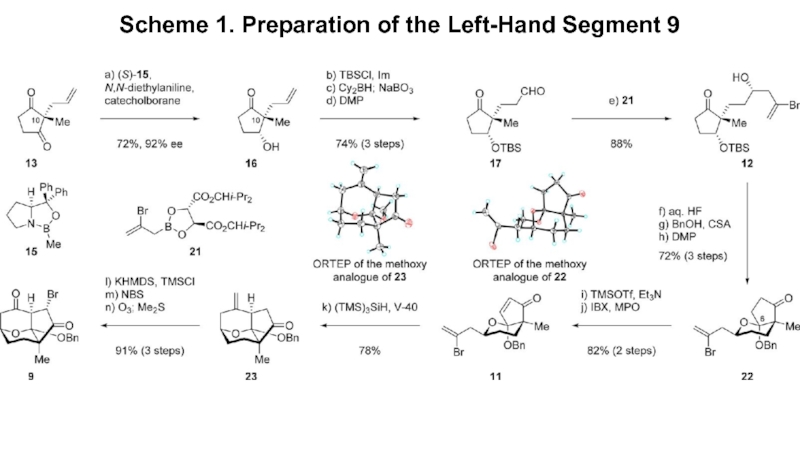
- 3. Scheme 1. Preparation of the Left-Hand Segment 9
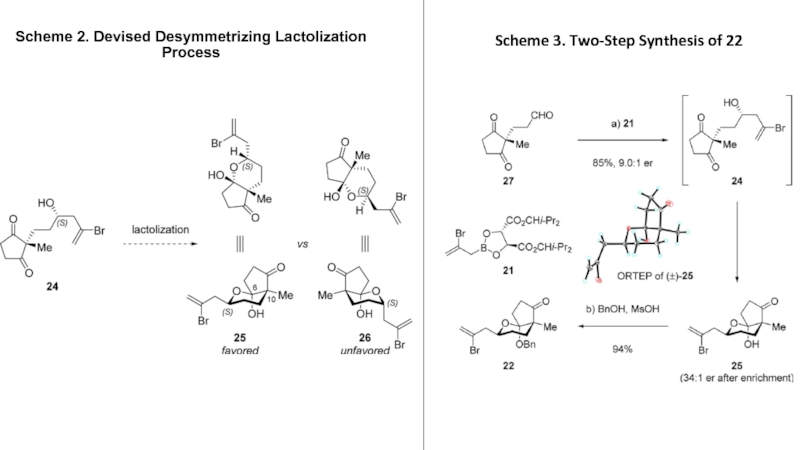
- 4. Scheme 2. Devised Desymmetrizing Lactolization ProcessScheme 3. Two-Step Synthesis of 22
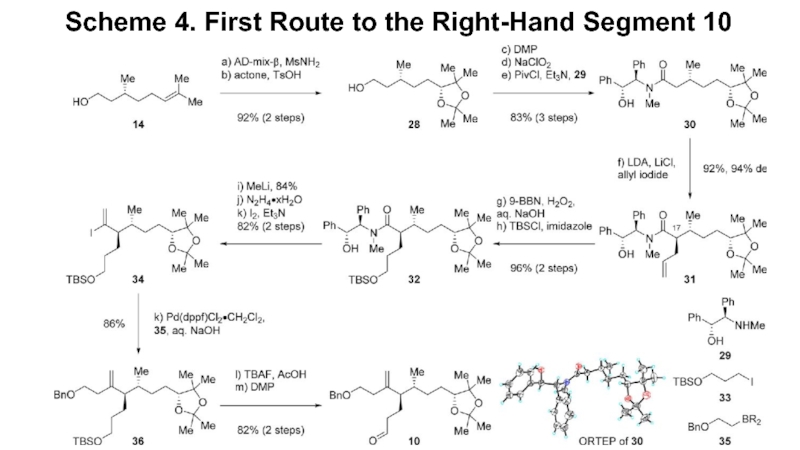
- 5. Scheme 4. First Route to the Right-Hand Segment 10
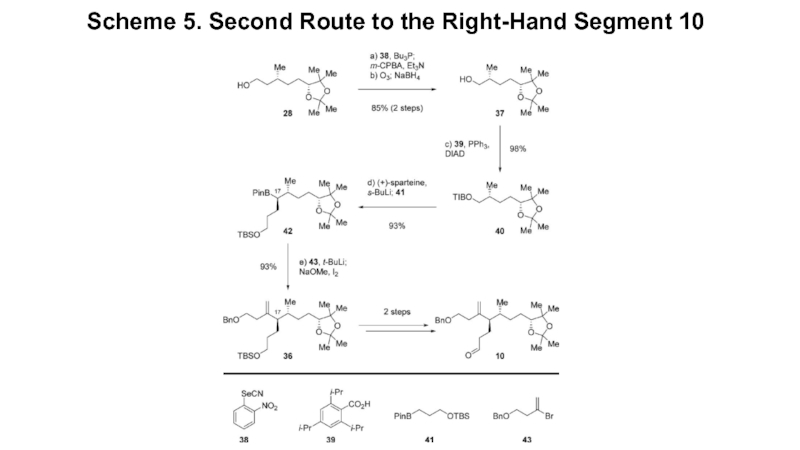
- 6. Scheme 5. Second Route to the Right-Hand Segment 10
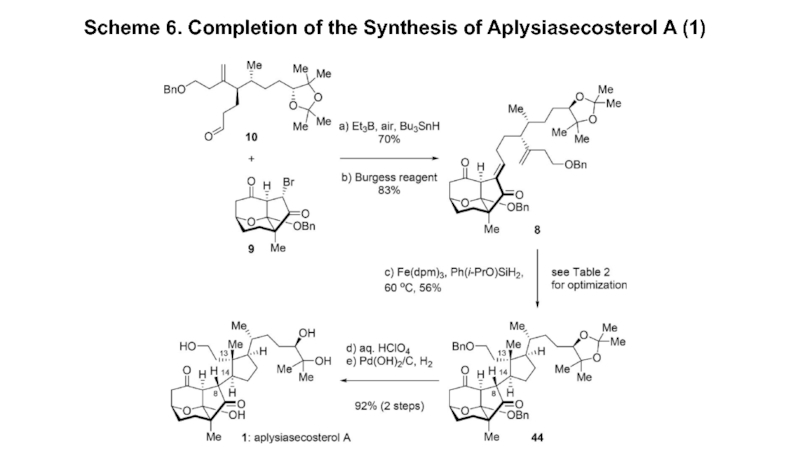
- 7. Scheme 6. Completion of the Synthesis of Aplysiasecosterol A (1)
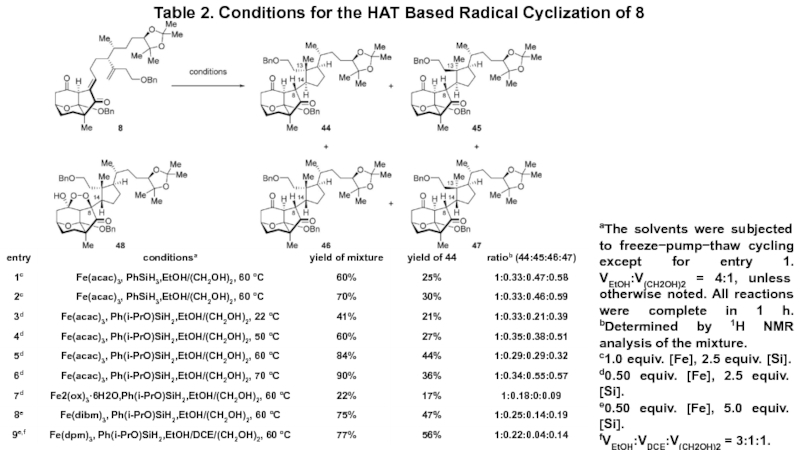
- 8. Table 2. Conditions for the HAT Based
- 9. Table 3. Cyclization of the Analogues of
- 10. Скачать презентанцию
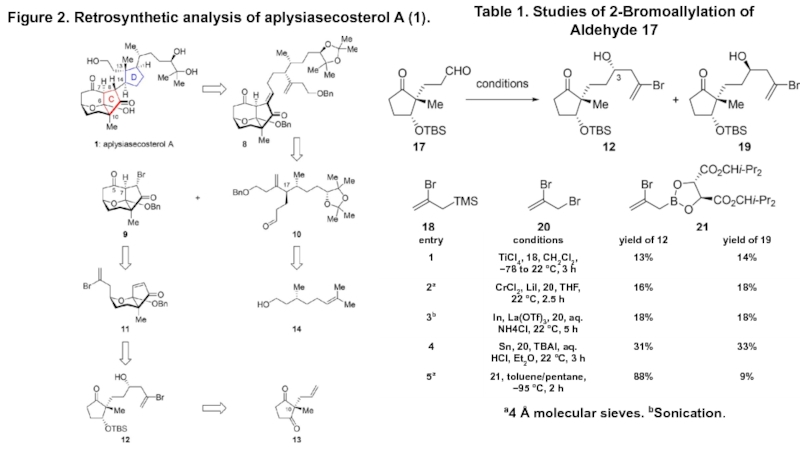
Figure 2. Retrosynthetic analysis of aplysiasecosterol A (1).Table 1. Studies of 2-Bromoallylation of Aldehyde 17a4 Å molecular sieves. bSonication.
Слайды и текст этой презентации
Слайд 1Total Synthesis of Aplysiasecosterol A
Figure 1. Aplysiasecosterol A (1) and
related secosteroids.
Слайд 2Figure 2. Retrosynthetic analysis of aplysiasecosterol A (1).
Table 1. Studies
of 2-Bromoallylation of Aldehyde 17
a4 Å molecular sieves. bSonication.
Слайд 8Table 2. Conditions for the HAT Based Radical Cyclization of
8
aThe solvents were subjected to freeze−pump−thaw cycling except for entry
1. VEtOH:V(CH2OH)2 = 4:1, unless otherwise noted. All reactions were complete in 1 h. bDetermined by 1H NMR analysis of the mixture. c1.0 equiv. [Fe], 2.5 equiv. [Si]. d0.50 equiv. [Fe], 2.5 equiv. [Si].
e0.50 equiv. [Fe], 5.0 equiv. [Si].
fVEtOH:VDCE:V(CH2OH)2 = 3:1:1.
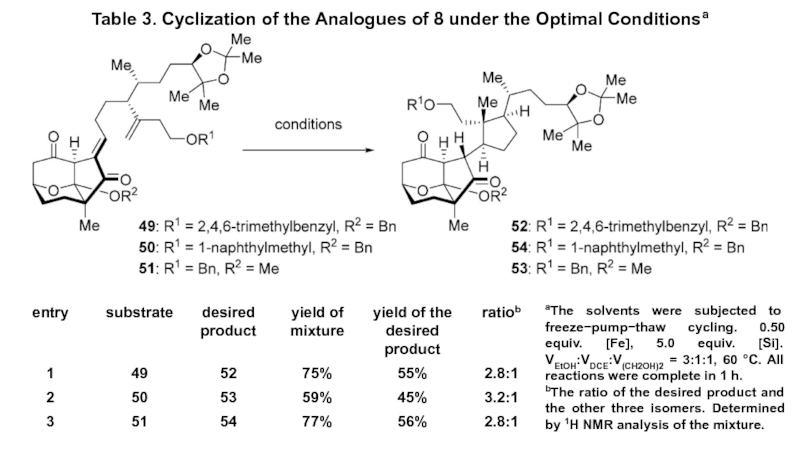
Слайд 9Table 3. Cyclization of the Analogues of 8 under the
Optimal Conditionsa
aThe solvents were subjected to freeze−pump−thaw cycling. 0.50 equiv.
[Fe], 5.0 equiv. [Si]. VEtOH:VDCE:V(CH2OH)2 = 3:1:1, 60 °C. All reactions were complete in 1 h. bThe ratio of the desired product and the other three isomers. Determined by 1H NMR analysis of the mixture.