Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Acidic environments Alkaline environments Review of biotechnologies Lecture
Содержание
- 1. Acidic environments Alkaline environments Review of biotechnologies Lecture
- 2. Слайд 2
- 3. Part 1. Acidic environmentsExtremely acidic environments have pH
- 4. Types of acidic environmentsHighly acidic habitats can
- 5. Species diversity in extreme acidic environments
- 6. Species diversity in extreme acidic environmentsElosa worallii
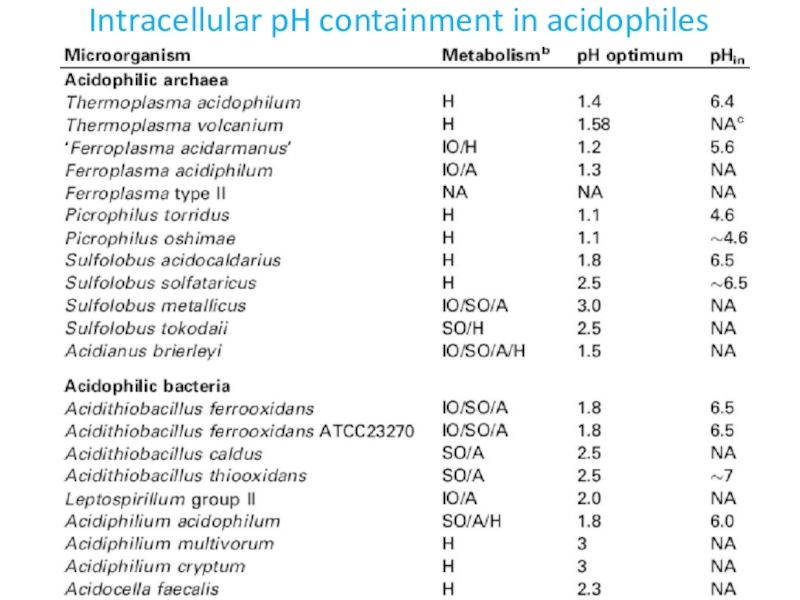
- 7. Intracellular pH containment in acidophiles
- 8. Metabolism and physiology of acidophilesAcidophiles seem to
- 9. Reversed membrane potentialA mechanism used by acidophiles
- 10. Excess protons are pumped out of the
- 11. The cell membrane is highly impermeable to
- 12. Cytoplasmic buffering and uncoupling of protonsIf H+
- 13. Upregulation of DNA/protein repair systems Acidophiles express
- 14. Protein folding on iron rivetsFerroplasma acidiphilum: an
- 15. The application of acidophiles: acetic acidAcetobacter aceti
- 16. Biodegradation of complex molecules: examplesAcidophiles have been
- 17. Other related biotechnologies with acidophilesBioremediation of iron
- 18. Alkaline environments Alkalinity is extremely common in
- 19. Part 2. Alkaline environments
- 20. Alkaline environments Soda lakes: the most stable
- 21. Primary producersDense blooms of cyanobacteria are responsible
- 22. Diversity of alkaliphiles Chemo-organotrophs: utilize and recycle
- 23. Diversity of alkaliphiles In the alkaline,hypersaline Mono
- 24. Mechanisms of adaptation to high alkalinityAlkaliphiles face
- 25. Mechanisms of adaptation to high alkalinityHowever, passive
- 26. AntiportersGram-positivePadan et al., 2011
- 27. AntiportersThis proton extrusion establishes a proton gradient
- 28. Mechanisms of adaptation to high alkalinityThe highly
- 29. Alkaliphiles in biotechnologyAlkaliphiles have evolved pH-stable enzymes
- 30. Part 3. Biotechnologies from extremophiles
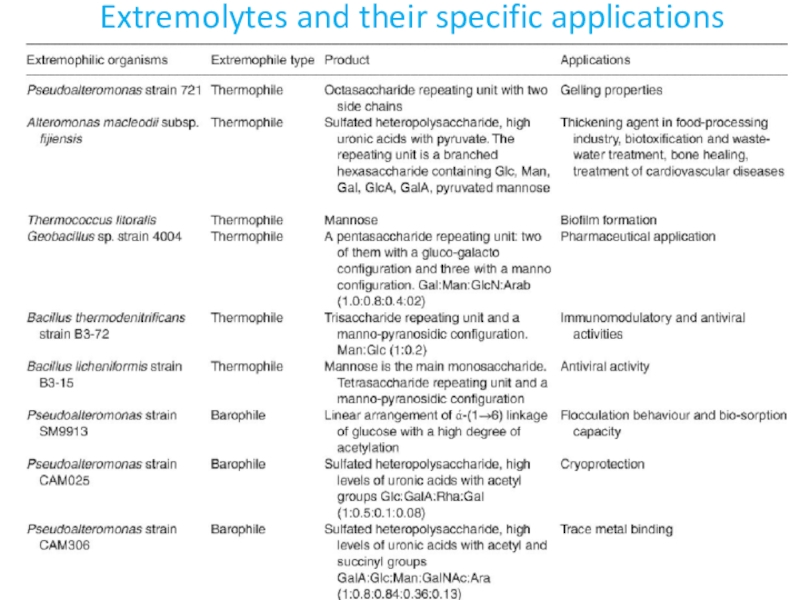
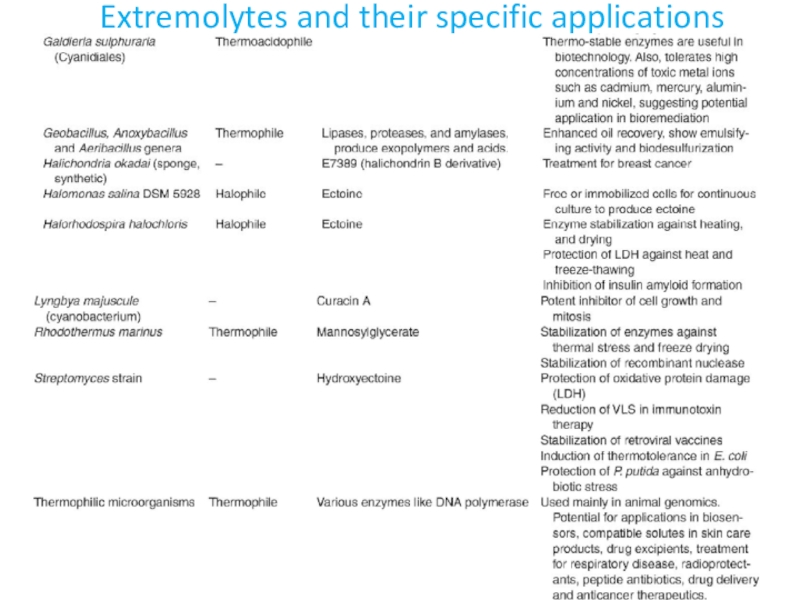
- 31. Extremolytes and their specific applications
- 32. Extremolytes and their specific applications
- 33. Extremolytes and their specific applications
- 34. Overview of applicationsThermophiles have widespread industrial and
- 35. Overview of applicationsAlkaliphilic microorganisms have made a
- 36. Extremophilic enzymes in biotechnologyA major constraint to
- 37. Extremophilic enzymes in biotechnologyAt the other end
- 38. Extremozymes in the detergent industryDetergent industry is
- 39. Extremozymes in biominingBiomining: extraction/recovery of precious and
- 40. Extremozymes in biorefining industryThermoacidophilic archaeon Sulfolobus solfataricus:
- 41. Extremozymes in the chemical industryThermotoga maritima: a
- 42. Extremozymes in pharmaceutical industry: examplesExtremozymes hold immense
- 43. Extremozymes in pharmaceutical industry: examplesHalophilic bacterium Ectothiorhodospira
- 44. Extremophiles in biosensingExtremophilic enzymes are used for
- 45. Extremophiles in biosensingChemical warfare agentsProlidase also has
- 46. Extremophiles in biosensingGlucose sensingDevelopment of a variety
- 47. Extremophiles in environmental applicationsLarge areas of soils,
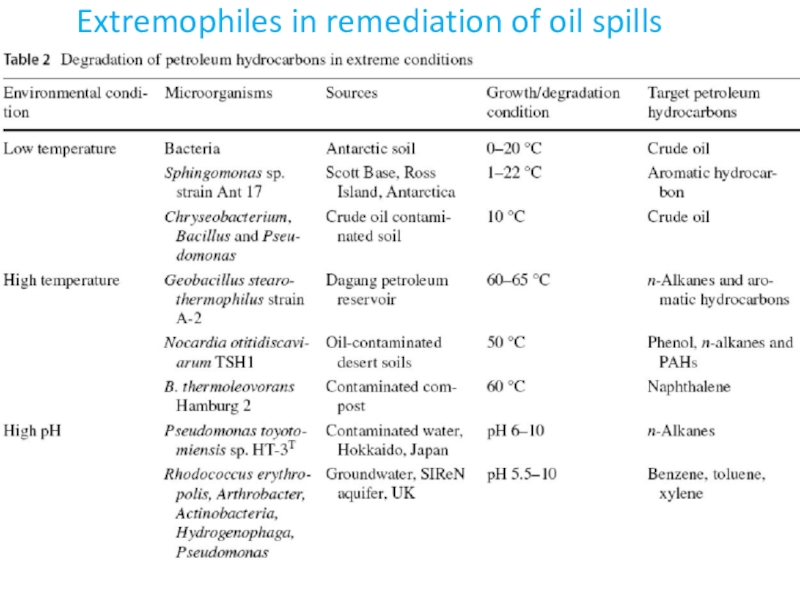
- 48. Extremophiles in remediation of oil spills
- 49. Extremophiles in remediation of oil spills
- 50. Bioremediation of radionuclidesIncreases in environmental radioactivity pose
- 51. Extremophiles as food additivesExtremophile enzymes play an
- 52. Скачать презентанцию
Слайды и текст этой презентации
Слайд 1Acidic environments
Alkaline environments
Review of biotechnologies
Lecture 15-16
Life under extreme conditions:
Слайд 3Part 1. Acidic environments
Extremely acidic environments have pH
persist
Many enzymes for basic metabolic processes cannot function
Only specialized organisms
can survive at low pHthe acidophiles and to some extent the acidotolerant species
prokaryotes, Archaea, and unicellular eukaryotes
To survive high [H+]ext and maintain a neutral intracellular pH, acidophiles had to evolve special adaptations
However, pH is not their only challenge
https://safesymptoms.com/stomach-spasm/
They are often subject to high temperatures
e.g. geysers in Yellowstone National Park
with temperature of 30-83°C and a pH of 2.7-3.7
They often also experience high levels of heavy metals (e.g. lead and zinc)
low pH is also often combined with high salinity of up to 23%
Слайд 4Types of acidic environments
Highly acidic habitats can be natural or
antropogenic
e.g. acidic mining lakes or acidic drainage
acidic volcanic lakes or
geysers in hot acidic geysers eukaryotes mainly inhabit the benthos (surface of sediments) or live within stone
How does the water in these environments become so acidic?
because sulfur/iron-oxidizing bacteria weather minerals rich in sulfur/iron
e.g. pyrite and marcasite;
The normally abiotic process
greatly accelerated by bacterial metabolism
The result of such weathering processes is accumulation of sulfuric acid and metal ions
That buffer and stabilize a low pH
pH is a decimal logarithm of the reciprocal of the H+ ion activity in a solution
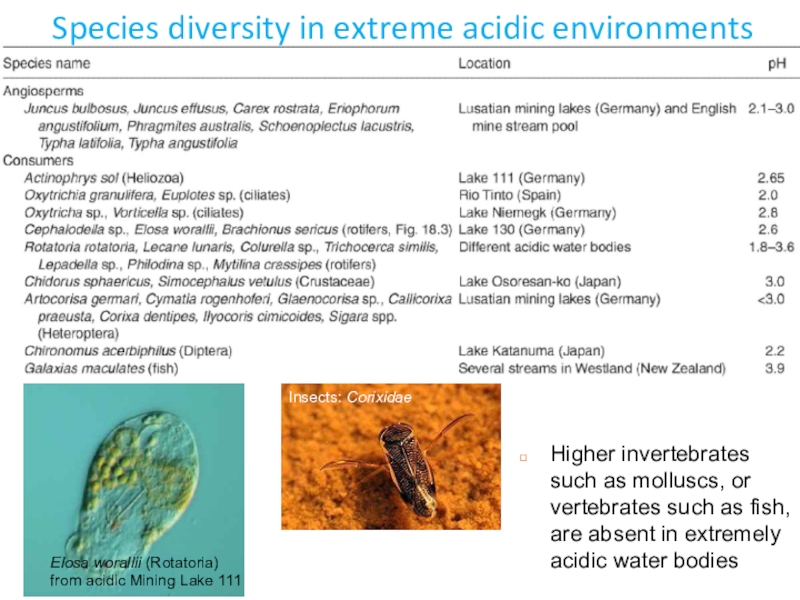
Слайд 6Species diversity in extreme acidic environments
Elosa worallii (Rotatoria) from acidic
Mining Lake 111
Insects: Corixidae
Higher invertebrates such as molluscs, or vertebrates
such as fish, are absent in extremely acidic water bodiesСлайд 8Metabolism and physiology of acidophiles
Acidophiles seem to share distinctive structural
and functional characteristics
A reversed membrane potential
Highly proton impermeable cell membranes
∆pH
is maintained through active proton export by transportersPredominance of secondary transporters
The presence and availability of enzymes and/or chemicals capable of binding and sequestering protons
More DNA/protein repair systems might be present in acidophiles than in neutrophiles
More effective degradation of organic acids that function as proton uncouplers
Слайд 9Reversed membrane potential
A mechanism used by acidophiles to reduce proton
influx is the generation of an inside positive TM potential
It is generated by a Donnan potential of positively charged molecules and inhibits the influx of protons using a chemiosmotic barrier against the proton gradient
This potential is possibly produced by a greater influx of potassium ions than the outward flux of protons
Not via channels but by cationic transporters
Difference from Nernst potential?
Слайд 10Excess protons are pumped out of the cell
Most acidophiles maintain
a near neutral intracellular pH
thereby have a pH gradient of
several pH units across the plasma membraneAcidophiles should use this TM ∆pH to generate large amounts of ATP!(?)
But this would result in rapid acidification of the cytoplasm
The protons that enter the cell through the F0/F1 ATPase need to be extruded
during electron transport and reduction of molecular oxygen at the terminal oxidase
and interference at any point in electron transport results in cessation of metabolism
Thus acidophiles express an abundance of H+ efflux systems (i.e. H+ ATPases, antiporters and symporters)
The numerous H+-driven secondary transporters
in Picrophilus torridus and Thermoplasma acidophilum the overall ratios of secondary to primary transporters in the genomes are 10:1 and 5.6:1, respectively
P. torridus lives in soil near a hot spring in Hokkaido, Japan The pH of the soil is less than 0.5
T. acidophilum was originally isolated from a self-heating coal refuse pile, at pH 2 and 59 °C
Plasma membrane H+-ATPase (P-type)
Слайд 11The cell membrane is highly impermeable to protons
To maintain ∆pH,
acidophiles need a highly H+ impermeable cell membrane
A balance between
H+ permeability, H+ influx through energetic and transport systems, and the rate of outward H+ pumping determines whether a cell can sustain an appropriate H+ motive force (PMF)An example of a highly impermeable cell membrane is the archaeal-specific structures composed of tetraether lipids
as opposed to the ester linkages found in bacterial and eukaryal cell membranes
Acidophilic bacteria accumulate saturated triacylglycerols
Triacylglycerols decrease membrane lipid fluidity
Their accumulation can prevent the osmotic imbalance caused by high [H2SO4]
https://ecampusontario.pressbooks.pub
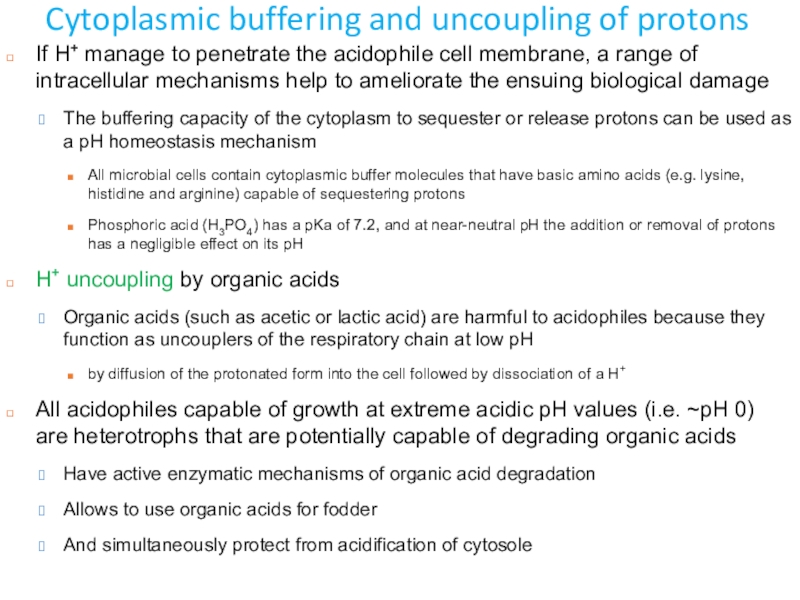
Слайд 12Cytoplasmic buffering and uncoupling of protons
If H+ manage to penetrate
the acidophile cell membrane, a range of intracellular mechanisms help
to ameliorate the ensuing biological damageThe buffering capacity of the cytoplasm to sequester or release protons can be used as a pH homeostasis mechanism
All microbial cells contain cytoplasmic buffer molecules that have basic amino acids (e.g. lysine, histidine and arginine) capable of sequestering protons
Phosphoric acid (H3PO4) has a pKa of 7.2, and at near-neutral pH the addition or removal of protons has a negligible effect on its pH
H+ uncoupling by organic acids
Organic acids (such as acetic or lactic acid) are harmful to acidophiles because they function as uncouplers of the respiratory chain at low pH
by diffusion of the protonated form into the cell followed by dissociation of a H+
All acidophiles capable of growth at extreme acidic pH values (i.e. ~pH 0) are heterotrophs that are potentially capable of degrading organic acids
Have active enzymatic mechanisms of organic acid degradation
Allows to use organic acids for fodder
And simultaneously protect from acidification of cytosole
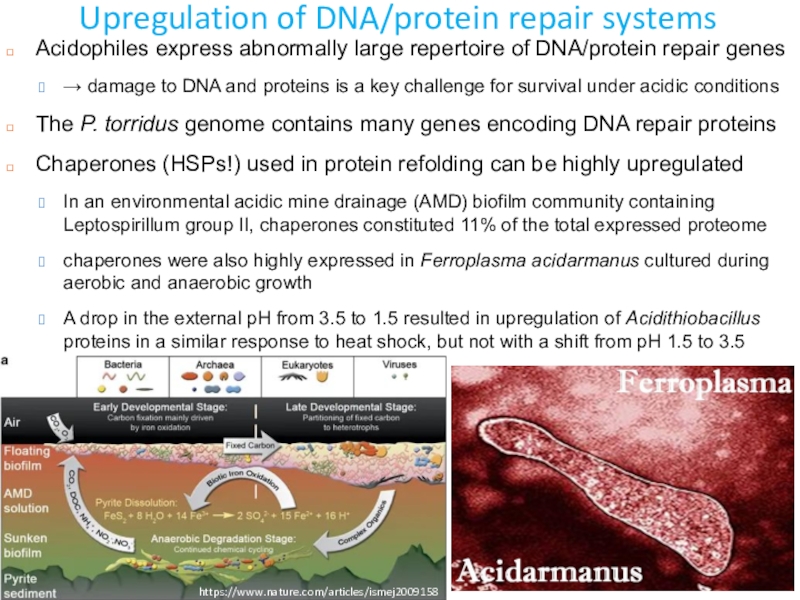
Слайд 13Upregulation of DNA/protein repair systems
Acidophiles express abnormally large repertoire
of DNA/protein repair genes
→ damage to DNA and proteins
is a key challenge for survival under acidic conditionsThe P. torridus genome contains many genes encoding DNA repair proteins
Chaperones (HSPs!) used in protein refolding can be highly upregulated
In an environmental acidic mine drainage (AMD) biofilm community containing Leptospirillum group II, chaperones constituted 11% of the total expressed proteome
chaperones were also highly expressed in Ferroplasma acidarmanus cultured during aerobic and anaerobic growth
A drop in the external pH from 3.5 to 1.5 resulted in upregulation of Acidithiobacillus proteins in a similar response to heat shock, but not with a shift from pH 1.5 to 3.5
https://www.nature.com/articles/ismej2009158
Слайд 14Protein folding on iron rivets
Ferroplasma acidiphilum: an autotrophic, iron-oxidizing archaeon
Obligate
acidophile, grows optimally at a pH of 1.7, at T
of ~35 °CFound in acidic mine tailings, primarily those containing pyrite (FeS2)
Abundant in cases of severe AMD, where other organisms such as Acidithiobacillus and Leptospirillum lower the pH to the extent that F. acidophilum can flourish
Lives by oxidizing Fe2+ in pyrite using oxygen as a terminal electron acceptor
This process produces H2SO4 as a by-product, further acidifying its environment
F. acidiphilum can grow at negative pH
Its enzymes can function at a much lower pH (1.7–4.0) than the predicted intracellular pH of 5.6
Due to intracellular compartmentalization of enzymes and presence of pH gradients (?)
Subsequently, it was found that the F. acidiphilum proteome contains a uniquely high proportion of iron proteins
Removal of the iron from 6 purified proteins resulted in the loss of secondary structure
Suggests that Fe is crucial in maintaining the protein folding, functioning as ‘iron rivets’
a potentially ancient property that stabilizes proteins retained in the archaeon
Слайд 15The application of acidophiles: acetic acid
Acetobacter aceti is a Gram-negative
flagellate bacterium
Basis of one of the most ancient biotechnologies:
fermentation of alcohol to acetic acidLives ubiquitously in alcoholic ecological niches
including flowers, fruits, etc., wherever sugar fermentation occurs
Grows optimally at 25 to 30°C; in pH ranging from 5.4 to 6.3, obligate aerobe
A. aceti is used for the mass production of vinegar
During the fermentation, it oxidizes ethanol in wines and ciders resulting in vinegar
Industrial production of acetic acid: ~10% by fermentation
Nowadays, most vinegar is made in submerged tank culture
In this method, alcohol is fermented to vinegar in a continuously stirred tank, and oxygen is supplied by bubbling air through the solution
Using modern applications of this method, vinegar of 15% acetic acid can be made in only 24 hours in batch process
and even 20% in 60-hour fed-batch process
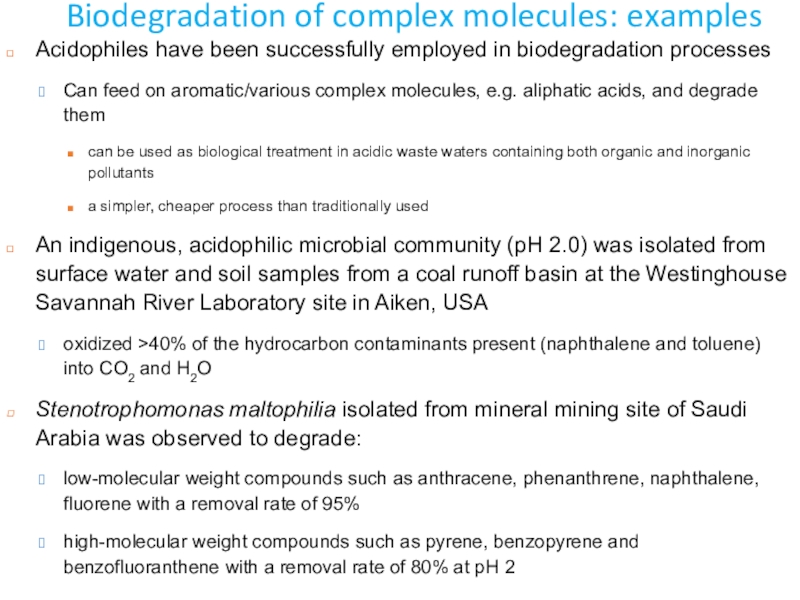
Слайд 16Biodegradation of complex molecules: examples
Acidophiles have been successfully employed in
biodegradation processes
Can feed on aromatic/various complex molecules, e.g. aliphatic acids,
and degrade themcan be used as biological treatment in acidic waste waters containing both organic and inorganic pollutants
a simpler, cheaper process than traditionally used
An indigenous, acidophilic microbial community (pH 2.0) was isolated from surface water and soil samples from a coal runoff basin at the Westinghouse Savannah River Laboratory site in Aiken, USA
oxidized >40% of the hydrocarbon contaminants present (naphthalene and toluene) into CO2 and H2O
Stenotrophomonas maltophilia isolated from mineral mining site of Saudi Arabia was observed to degrade:
low-molecular weight compounds such as anthracene, phenanthrene, naphthalene, fluorene with a removal rate of 95%
high-molecular weight compounds such as pyrene, benzopyrene and benzofluoranthene with a removal rate of 80% at pH 2
Слайд 17Other related biotechnologies with acidophiles
Bioremediation of iron and sulfate-rich lakes
By
reducing iron and sulfur, and alkalizing the environment after mining
preventing
acidification by inhibiting growth of Fe/S-oxydizing prokaryotes, e.g. Acidithiobacillus ferrooxidansBiomining/bioleaching, a process opposite to bioremediation
Extraction of metals from ores (especially depleted and sulfide-rich) and other solid materials
using Acidithiobacillus ferrooxidans and similar species
utilized when conventional mining procedures are too expensive or ineffective in recovering a metal such as copper, cobalt, gold, lead, nickel, uranium and zinc
Слайд 18Alkaline environments
Alkalinity is extremely common in inland waterways worldwide
80%
are on the alkaline side of neutrality
Extremely alkaline environments
both manmade and naturally occurring are not restricted to aquatic systems Can be found in a wide range of geographic locations
Bacillus bacteria and the alkaliphilic mycelial prokaryotes Streptornyces and Nocardiopsis grow particularly well in various alkaline environments
Слайд 20Alkaline environments
Soda lakes: the most stable high-pH environments on
Earth
Soda lakes contain alkaline brine enriched in CO32- and Cl-
Their pH can range from 8 to >12
Akin to the pH of many household cleaning products
Scarcity of Mg2+ and Ca2+ in the strata surrounding soda lakes and in the water
Low Mg2+ and Ca2+ required for formation
The majority of these microorganisms are alkaliphiles
Grow very well at pH >8, usually with optima at pH 9-10, and sometimes at pH >12
Another major physiological group in soda lakes are haloalkaliphiles
require both alkaline conditions (>pH 9), and high, up to 33% NaCl for growth
Other polyextremophiles include the thermoalkaliphiles
grow at elevated temperatures (>60°C) and pH 9, e.g. Thermus brockianus
Soda lakes are characterized by very high productivity
at rates exceeding 10 g m-2 day-1.
Due to the relatively high ambient temperatures and light intensities, available phosphate and unlimited CO2 in the carbonate-rich waters
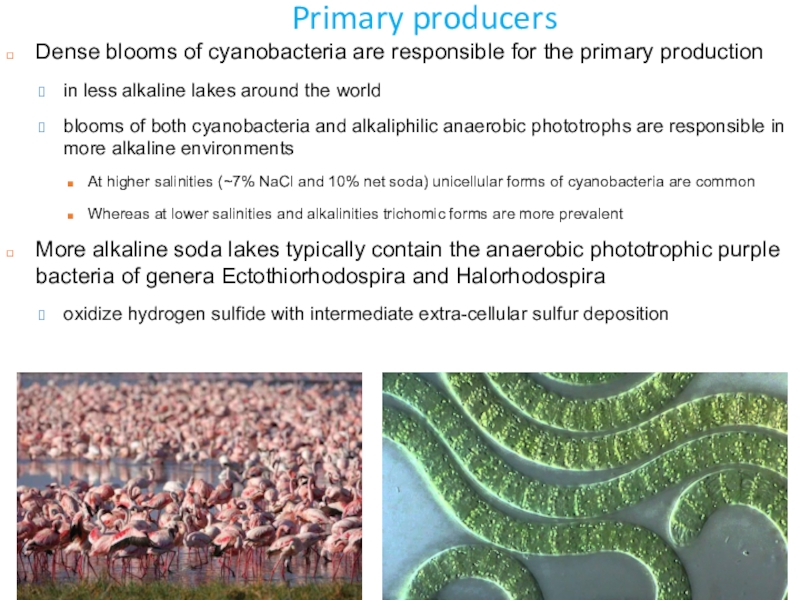
Слайд 21Primary producers
Dense blooms of cyanobacteria are responsible for the primary
production
in less alkaline lakes around the world
blooms of both cyanobacteria
and alkaliphilic anaerobic phototrophs are responsible in more alkaline environments At higher salinities (~7% NaCl and 10% net soda) unicellular forms of cyanobacteria are common
Whereas at lower salinities and alkalinities trichomic forms are more prevalent
More alkaline soda lakes typically contain the anaerobic phototrophic purple bacteria of genera Ectothiorhodospira and Halorhodospira
oxidize hydrogen sulfide with intermediate extra-cellular sulfur deposition
Слайд 22Diversity of alkaliphiles
Chemo-organotrophs: utilize and recycle the products of
photosynthesis
Various aerobic, anaerobic, alkaliphilic, haloalkaliphilic, thermophilic etc. procaryotes
Methanogens: recycle
methane C/H into the common pool of organic matterSulfur-reducing and -oxidizing alkaliphiles
Most soda lakes contain black muds due to sulfidogenesis
Alkaliphilic, sulfate-reducing bacteria play an important role as hydrogen sinks as they utilize hydrogen as an electron donor
Acetogenic and acetoclastic alkaliphiles:
The production and use of acetate promotes cross-feeding in alkaliphilic microbiomes
Nitrogen-fixing, ammonia- and nitrite-oxidizing, and nitrate-reducing alkaliphiles: most alkaliphiles contribute
Fungi: lipases from Fusarium solani (China) are used in detergents
Protozoa
Viruses
Animalia: Alkaline environments support very few species of invertebrate, although the densities of those that they do support can be extremely high
Слайд 23Diversity of alkaliphiles
In the alkaline,hypersaline Mono Lake, USA, only
2 stable invertebrates live
The brine shrimp, Artemia monica
15-17 individuals L-1
in nearshore regions; 6-8 individuals L-1 in the pelagicThe alkali fly, Ephydra hians
up to 100 g dry weight m-2
Female alkali flies walk down a substrate and lay their eggs on algae mats at depths of ~3 m
Слайд 24Mechanisms of adaptation to high alkalinity
Alkaliphiles face many challenges in
an alkaline environment and evolved a range of strategies to
survivemaintain internal pH > 2 units lower than the external environment
Bacteria of the genera Bacillus are the most well studied alkaliphiles
they achieve pH homeostasis via both passive and active regulation mechanisms
The cell surface is key in discriminating the internal from external environment and maintaining the intracellular neutral environment
Maintaining a negatively charged cell wall is a very effective mechanism
The cell walls of several alkaliphilic Bacillus spp. contain acidic polymers
galacturonic acid, gluconic acid, glutarnic acid, aspartic acid and phosphoric acid etc.
These acidic macromolecules provide a passive barrier to ion flux
Their negative charges help adsorb sodium and hydronium (H3O+) ions and repulse OH- e
When these acidic residues are lost due to mutations, ability to grow in alkaline conditions is severely diminished
Слайд 25Mechanisms of adaptation to high alkalinity
However, passive methods of cytosolic
acidification alone are not sufficient to maintain an internal pH
by 2.0-2.3 points below external pHThere must also be active forms of acidification
E. coli can alter its metabolism to generate acids in the cytoplasm in response to high external pH by
up-regulating deaminases, ATP synthase, and the microaerophilic cytochrome d oxidoreductase
A major strategy for bacterial pH homeostasis is the use of transporters that catalyze active proton transport
resulting in the efflux of intracellular monovalent cations (such as Na+, K+, and Li+) in exchange for external protons
This process reduces the cytoplasmic concentration of toxic cations
supports Na+/K+-dependent cytoplasmic pH homeostasis under alkaline conditions
The best characterized method of active acidification is by Na+/H+ antiporters
H+ ions are first extruded through the electron transport chain in respiring cells
and to some extent through an ATPase in fermentative cells
Слайд 27Antiporters
This proton extrusion establishes a proton gradient that drives electrogenic
antiporters
intracellular Na+ removed out of the cell in exchange for
a greater number of H+ ionsleads to the net accumulation of internal protons
This proton accumulation leads to a lowering of cytosolic pH
The extruded Na+ can be used for the symport of other solutes
Examples:
the coupling stoichiometry of Ec-NhaA is 2H+/1Na+
The stoichiometry of Ec-NhaB is 1.5H+/1Na+
This small difference in electrogenicity underpins the ability of Ec-NhaA but not Ec-NhaB to support growth of E. coli at alkaline pH in the presence of Na+
If Na+/H+ antiporters are disabled through mutation or another means, the bacteria lose the ability to survive in high pH
The sodium required for this antiport system is the reason some alkaliphiles can only grow in saline environments
Слайд 28Mechanisms of adaptation to high alkalinity
The highly alkali environment necessitates
differences in ATP production
Generally, ATP production operates by establishing a
proton gradient greater H+ concentration outside the membrane
and a transmembrane electrical potential
with a positive charge outside the membrane)
However, since alkaliphiles have a reversed pH gradient, it would seem that ATP production based on a strong H+ motive force would be severely reduced
However, the opposite is true
It appears that while the pH gradient seems to be reversed, the TM electrical potential is greatly increased
This increase in charge causes the production of greater amounts of ATP by each translocated proton when driven through an ATP synthase
Слайд 29Alkaliphiles in biotechnology
Alkaliphiles have evolved pH-stable enzymes resistant to the
effects of extreme pH and able to remain active in
the absence of Mg2+ and Ca2+A large number of enzymes from alkaliphiles were studied and many now are used inindustrial applications
including detergent additives, hide de-hairing, food processing, recovery of silver from X-ray films, production of cyclodextrins for foodstuffs, chemicals and pharmaceuticals, biological bleaching of wood pulp, and waste treatments
Alkaline Morning Glory Prismatic Hot Spring, Yellowstone National Park, USA
Слайд 34Overview of applications
Thermophiles have widespread industrial and pharmaceutical applications
Psychrophiles have
been used to obtain cold-active enzymes
Used in the detergent and
food industriesIn specific biotransformations and environmental bioremedialions
Specialized uses in contact lens cleaning fluids
Reducing the lactose content of milk
Anti-freeze proteins have potential uses in the manufacture of ice cream
Ice-nucleating proteins have potential uses in the manufacture of artificial snow
Lipids isolated from Antarctic marine psychrophiles are used as dietary supplements in the form of polyunsaturated fatty acids
Barophiles have yielded several natural products of potential use in human health and environmental bioremediation
piezophiles can potentially be sources of novel restriction endonucleases or other DNA-binding proteins
Слайд 35Overview of applications
Alkaliphilic microorganisms have made a significant widespread impact
on
food processing
potassium hydroxide-mediated removal of potato skins
cement manufacture, alkaline
electroplating, leather tanning, paper and board manufacture, indigo fermentation, rayon manufacture, herbicide manufacture etc.Among halophiles, the Archaeon Halobacteria salinarum produces the photosynthetic protein bacteriorhodopsin
and the alga Dunaliella is used for the commercial production of β-carotene
Other halophiles are used to produce polymers (e.g. polysaccharides), enzymes, and compatible solutes
Enhancing processes such as oil recovery, cancer detection, drug screening and the biodegradation of residues and toxic compounds
Acidophiles are used begin to be used in metallurgy
Слайд 36Extremophilic enzymes in biotechnology
A major constraint to the biotechnological application
of mesophilic enzymes is their low stability to heat, pH,
organic solvents and proteolytic degradationExtremozymes can remain stable under extreme physical/chemical stresses
Plus they often have higher reaction rates,
or the capability of destroying and/or eliminating xenobiotics,
or the ability to modulate the hyper-accumulation of substances such as heavy metals, pollutants and radionuclides
Discovery and isolation of Taq DNA polymerase from T. aquaticus was a turning point
Its use revolutionized PCR DNA amplification technique
eliminated the need to add enzyme after every cycle of thermal denaturation of the DNA
PCR is now a common, indispensable widely used technique
DNA cloning for sequencing, DNA-based phylogeny, functional analysis of genes, diagnosis of hereditary and infectious diseases, the detection of contaminants in forensics, and for as agricultural uses etc.
Taq polymerase is also used for numerous other applications in research and industry
Слайд 37Extremophilic enzymes in biotechnology
At the other end of the T
scale are the enzymes isolated from psychrophiles
Such as lipases,
proteases and cellulases have been used as additives in detergents for work at low temperature, or as additives in the frozen food industryCold-active lipases maintain high catalytic activity at low temperatures and thus have a broad spectrum of biotechnological applications
e.g. cold active lipases isolated from the fungus Candida Antarctica have been patented for use in industrial biocatalysis
Chinese plant
From alkaliphiles: Cao et al. (1992) isolated four alkaliphilic bacteria, NT-2, NT-6, NT-33 and NT-82, producing pectinase and xylanase
NT-33 has an excellent capacity for degumming ramie (Boehrneria nivea) fibres
increasingly used in the textile industry to produce high strength ramie-cotton fabric composites
Similarly, peptic lyase produced by the alkaliphilic Bacillus sp. strain GIR 277, has been used to improve the production of a type of Japanese paper
Слайд 38Extremozymes in the detergent industry
Detergent industry is the largest market
for enzymes (25-30% of total sales)
Over half of all
detergents presently available contain extremozymes Proteases, amylases, cellulases, lipases etc.
Detergent formulations routinely contain 0.4-0.8% of crude enzymes by weight
Of particular importance was the discovery of alkaliphilic-based proteolytic enzymes, serine proteases, characterized by
(i) long term stability, (ii) energy and cost-efficiency, (iii) quicker and more reliable product, (iv) reduced effluents, (v) stability in the presence of detergent additives such as bleach activators, softeners, bleaches and perfumes
Biotex® detergent launched in 1960s contained an alkaline proteinase from bacterium Bacillus licheniformis
since then a large number of enzymes that function very efficiently in solution at a pH of between 8 and 10.5 were isolated
alginases, alkaline proteases, amylases, α-galactosidase, β-galactosidase, cellulases, catalases, DNases, glucimases, pectinases, etc. etc.
Слайд 39Extremozymes in biomining
Biomining: extraction/recovery of precious and base metals from
mineral ores and concentrates using extremophilic microorganisms
Developed into a
successful and expanding area of biotechnology in recent years has distinctive advantages over traditional mining
Two main processes are used: biooxidation and bioleaching
Biooxidation is a microbially-mediated oxidation process in which the valuable metals remain in the solid phase but become enriched
Bioleaching is a contrasting method as a valuable metal is solubilized in water filtered through mineral ores as a result of metabolism of bioleaching microbes
The metals are subsequently recovered from the leachate
Biomining appears to require less capital, lower operating costs, and less skilled operating and maintenance personnel than traditional techniques
Слайд 40Extremozymes in biorefining industry
Thermoacidophilic archaeon Sulfolobus solfataricus:
Active at 55-90°C
and pH 0.9-5.8, with optimal pH 2 to 4, and
cytoplasmic pH of 6.5Either oxidizes sulfur, or metabolizes various carbohydrates with the help of sulfur
Auto-aggregate when exposed to UV light, creating multi-cellular structures
S. solfataricus is used in the saccharification step in starch processing to obtain glucoamylase
involves hydrolysis of oligosaccharides into glucose/glucose syrups
Thermophilic, mildly halophilic bacterium Rhodothermus marinus
heterotrophic obligate aerobe
Grows in the range of 54-77°C, with optimal 65°C; at NaCl concentrations optimally at 2% and not above 6%; with optimum pH for growth of 7.0
Its xylanase is used to improve brightness in the bleaching sequences of hard- and softwood pulps prepared by Kraft processing at 80°C
The conversion of wood into wood pulp consisting of almost pure cellulose fibres
Use of the xylanase increases the process rate and eliminating the use of toxic chlorine for bleaching
Слайд 41Extremozymes in the chemical industry
Thermotoga maritima: a hyperthermophilic anaerobic bacterium,
grows in the geothermal sediment in the range of 55–90°C,
with an optimum at 80°Cthe only bacterium known to grow at such high temperature
catabolizes sugars/polymers, producing CO2 and H2 as fermentation by-products
Can reduce Fe(III) to produce energy using anaerobic respiration
Its aldo-keto reductase enzyme is stable up to 80°C and retains over 60% activity for 5 h at this temperature
Proved useful in the production of primary alcohols from substrates such as benzaldehyde, 1,2,3,6-tetrahydrobenzaldehyde and para-anisaldehyde
Furthermore, a novel thymidine kinase (TmTK) shows high substrate specificity at the native growth temperature of 82°C but turns promiscuous at 37°C
Researched to increase the general understanding of substrate promiscuity among extremozymes
Слайд 42Extremozymes in pharmaceutical industry: examples
Extremozymes hold immense promise for the
pharmaceutical industry
Many pharmaceutically active compounds have already been isolated
antibacterial,
antialgal, antihelminthic, antivirals, antiprotozoals, immunomodulatory, anticancer, cardioprotective, antioxidants, anticoagulants, radioprotective etc.Antioxidants and anticancer compounds:
Acidophilic green microalga Chlamydomonas acidophila from Tinto River
When glycine is supplied as a carbon source, the alga accumulates high concentrations of lutein (~10 g kg-1 of dry weight)
and produces large amounts of zeaxanthin, a lutein’s isomer
Lutein: a carotenoid xanthophyll
in the human retina, lutein is absorbed from blood specifically into the macula lutea
Both lutein and zeaxantin are widely used in food and supplement manufacturing as colorants
The pharmaceutical market for lutein is ~$190 million; the food-related categories market ~$110 million; pet food and other animal applications for lutein ~US$175 million annually
Слайд 43Extremozymes in pharmaceutical industry: examples
Halophilic bacterium Ectothiorhodospira halochloris produces ectoine
An
extremely halophilic purple bacterium found proliferating in saturated salts
e.g.
in crystallizer ponds, an environment where NaCl precipitates, at >25% of NaClEctoine is a small organic molecule
also found in a wide range of Gram-negative and Gram-positive bacteria
by balancing the osmotic pressure, protects the extremophiles against the dehydration caused by high temperatures, high salt concentrations and low water activity in their native environment
Used as an active ingredient in skin care and sun protection products
Stabilizes proteins and other cellular structures and protects the skin from stresses like UV irradiation and dryness
Marketed as an anti-ageing medicine
E. halochloris also has a strong potential for photobiological hydrogen generation as a byproduct of nitrogen fixation through nitrogenase
hydrogen fuel source
also used/researched for the production of glycine, betaine, and trehalose
Слайд 44Extremophiles in biosensing
Extremophilic enzymes are used for the development of
simple and highly sensitive biosensors
e.g. thermophilic enzymes have been
used for the construction of optical nanosensors, stable and non-consuming analytesBased on the ability of thermophilic enzymes to bind the substrate at room temperature without transforming it
The binding of substrate can be monitored by fluorescence variation of the enzyme
Fluorescence detection, due to simplicity and sensitivity, is now the dominant analytical tool in medical testing, biotechnology and drug discovery
Cancer detection
Prolidase is a ubiquitous peptidase isolated from a number of extremophiles, in particular the hyperthermophilic archaeon Pyrococcus furiosus
Prolidase specifically hydrolyses dipeptides with a prolyl residue in the carboxyl terminus (NH2-X-/-Pro-COOH)
Increased prolidase activity in melanoma cell lines has led investigators to create cancer prodrugs targeting this enzyme
Слайд 45Extremophiles in biosensing
Chemical warfare agents
Prolidase also has the ability to
degrade toxic organophosphorus (OP) compounds by cleaving the P-F and
P-O bonds in the nerve agents sarin and somanSarin attacks the nervous system by interfering with the degradation of the acetylcholine at neuromuscular junctions
Death will usually occur as a result of asphyxia due to inability to breath
The applications of prolidase for detoxifying OP nerve agents include its incorporation into firefighting foams and as biosensors for OP compound detection
Sarin
Слайд 46Extremophiles in biosensing
Glucose sensing
Development of a variety of diabetic health
care devices is a long-standing research priority
continuous, noninvasive, painless glucose
monitoring, control of an insulin pump and a warning system for hyper- and hypoglycaemic conditions A thermostable glucokinase from the thermophilic bacterium Bacillus stearothermophilus is tested for use as a reversible glucose sensor and employed in glucose assays
The bacterium is also used as a challenge organism for sterilization validation studies and periodic check of sterilization cycles
Glucose dehydrogenase (GD) from the thermoacidophilic archaeon Thermoplasma acidophilum can also be employed in glucose sensing because it can function in the absence of a coenzyme
Слайд 47Extremophiles in environmental applications
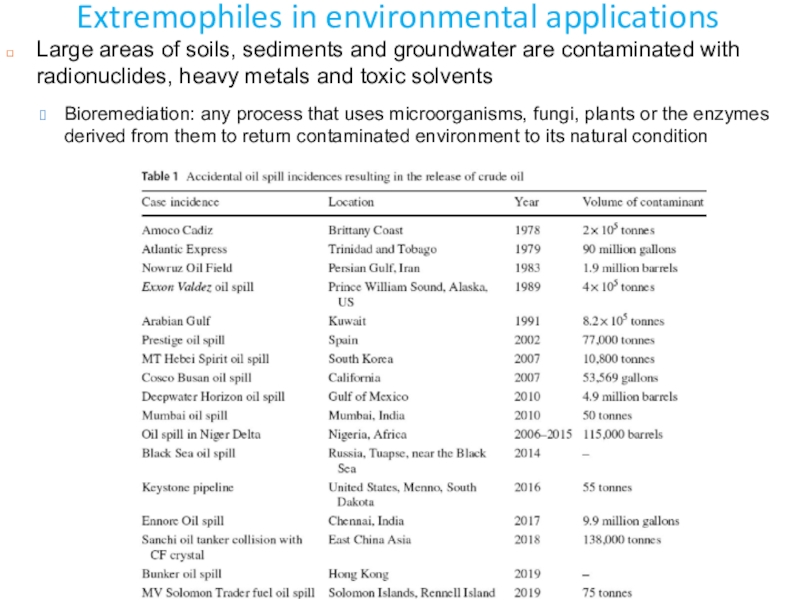
Large areas of soils, sediments and groundwater
are contaminated with radionuclides, heavy metals and toxic solvents
Bioremediation: any
process that uses microorganisms, fungi, plants or the enzymes derived from them to return contaminated environment to its natural condition Слайд 50Bioremediation of radionuclides
Increases in environmental radioactivity pose a persistent danger
Radionuclides can enter the food chain
The high cost of
remediating radioactive waste sites from nuclear weapons production stimulates development of bioremediation strategiesBioremediation in the case of nuclear waste, ionizing radiation limits the amount of microorganisms that can be useful
Radioresistant thermophilic bacterium Deinococcus radiodurans is used for the treatment of mixed radioactive wastes containing ionic mercury
D. radiodurans has been genetically engineered to consume and digest solvents and heavy metals in radioactive environments
The mercuric reductase gene was cloned from E. coli into D.r. to detoxify ionic mercury found in radioactive waste from nuclear weapons manufacture
Moreover, a gene encoding a non-specific acid phosphatase from Salmonella enterica serovar Typhi was cloned into D.r. for bioprecipitation of uranium in acid solutions
The alkaline phosphatase gene from Sphingomonas have been introduced into D.r. for bioprecipitation of uranium in alkaline solutions
Reduce a variety of radionuclides to less mobile, less toxic compounds
Слайд 51Extremophiles as food additives
Extremophile enzymes play an important role in
the food industry
Extremozymes from marine organisms are widely used
Enzymes from extremophilic fish and marine microorganisms are superior to the traditional enzymes due to their ability to function at extremes of temperature and pH
Psychrophilic fish proteins, e.g. collagens and their gelatin derivatives, are stable at low temperatures
Used in heat-sensitive processes such as gelling and clarifying
Psychrophilic hydrolases such as β-glucanases, cellulases, pectinases and proteinases find application in the food industry
A thermostable polygalacturonase from a thermophilic mould, Sporotrichum triermophile, when added to fruit pulps with xylanase and cellulose, increases the yield of fruit juices
The halophilic, lactic acid bacterium, Tetragenococcus halophila, is used in soy sauce production via fermentation that involves high salt concentration
Other halophilic bacteria employed include Lactobacillus planetarium, H. salinarum, Halococcus spp. and Bacillus spp.