Слайд 1HBV
Clinical Practice Guidelines
Слайд 2About these slides
These slides give a comprehensive overview of the
EASL clinical practice guidelines on the management of hepatitis B
infection
The guidelines were published in full in the August 2017 issue of the Journal of Hepatology
The full publication can be downloaded from the
Clinical Practice Guidelines section of the EASL website
Please cite the published article as: European Association for the Study
of the Liver. EASL 2017 Clinical Practice Guidelines on the management
of hepatitis B virus infection. J Hepatol 2017;67:370–98
Please feel free to use, adapt, and share these slides for your own personal use; however, please acknowledge EASL as the source
Слайд 3About these slides
Definitions of all abbreviations shown in these slides
are provided within the slide notes
When you see a home
symbol like this one: , you can click on
this to return to the outline or topics pages, depending on which
section you are in
Please send any feedback to: slidedeck_feedback@easloffice.eu
These slides are intended for use as an educational resource and should not be used in isolation to make patient management decisions. All information included should be verified before treating patients or using any therapies described in these materials
Слайд 4Chair
Pietro Lampertico
Panel members
Kosh Agarwal, Thomas Berg, Maria
Buti, Harry LA Janssen,
George Papatheodoridis, Fabien Zoulim, Frank Tacke
(EASL Governing Board representative)
Reviewers
Maurizia Brunetto, Henry Chan, Markus Cornberg
Guideline panel
EASL CPG HBV. J Hepatol 2017;67:370–98
Слайд 5Outline
EASL CPG HBV. J Hepatol 2017;67:370–98
Слайд 6Methods
Grading evidence and recommendations
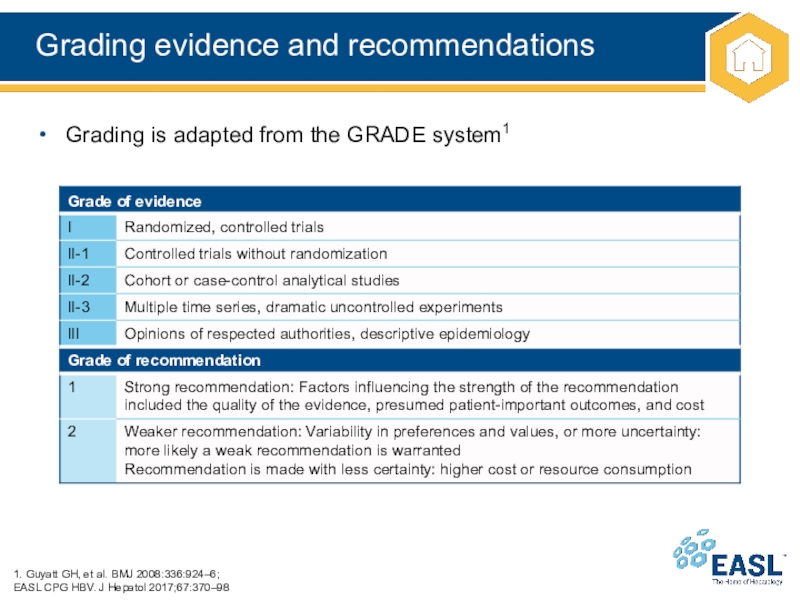
Слайд 7Grading evidence and recommendations
1. Guyatt GH, et al. BMJ
2008:336:924–6;
EASL CPG HBV. J Hepatol 2017;67:370–98
Grading is adapted from the
GRADE system1
Слайд 8Background
Epidemiology of HBV
New nomenclature for chronic phases
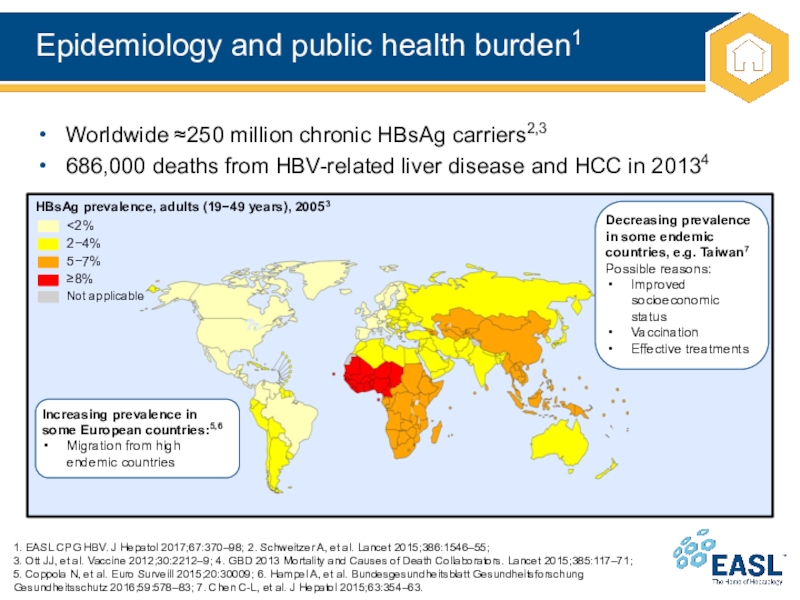
Слайд 9Epidemiology and public health burden1
1. EASL CPG HBV. J Hepatol
2017;67:370–98; 2. Schweitzer A, et al. Lancet 2015;386:1546–55;
3. Ott
JJ, et al. Vaccine 2012;30:2212–9; 4. GBD 2013 Mortality and Causes of Death Collaborators. Lancet 2015;385:117–71;
5. Coppola N, et al. Euro Surveill 2015;20:30009; 6. Hampel A, et al. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2016;59:578–83; 7. Chen C-L, et al. J Hepatol 2015;63:354–63.
Worldwide ≈250 million chronic HBsAg carriers2,3
686,000 deaths from HBV-related liver disease and HCC in 20134
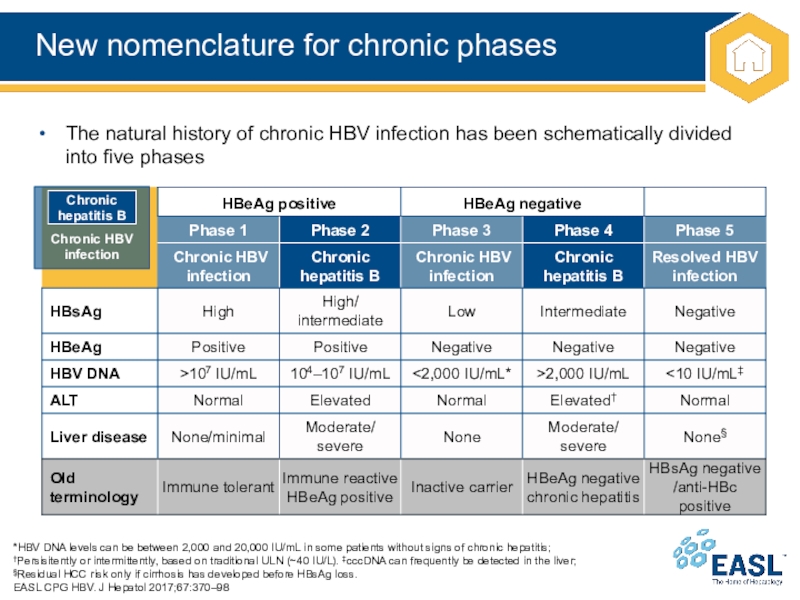
Слайд 10New nomenclature for chronic phases
*HBV DNA levels can be between
2,000 and 20,000 IU/mL in some patients without signs of
chronic hepatitis;
†Persisitently or intermittently, based on traditional ULN (~40 IU/L). ‡cccDNA can frequently be detected in the liver;
§Residual HCC risk only if cirrhosis has developed before HBsAg loss.
EASL CPG HBV. J Hepatol 2017;67:370–98
The natural history of chronic HBV infection has been schematically divided into five phases
Chronic HBV
infection
Chronic hepatitis B
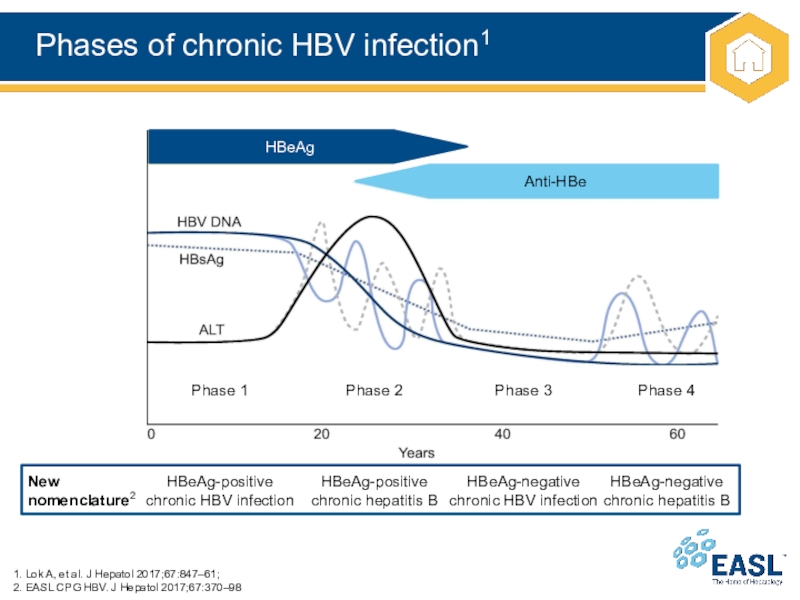
Слайд 11Phases of chronic HBV infection1
1. Lok A, et al. J
Hepatol 2017;67:847–61;
2. EASL CPG HBV. J Hepatol 2017;67:370–98
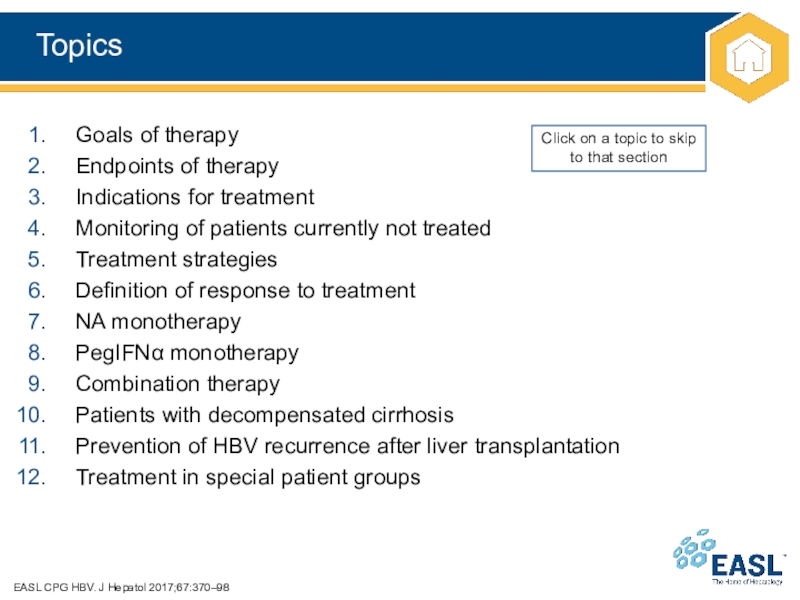
Слайд 13Topics
EASL CPG HBV. J Hepatol 2017;67:370–98
Goals of therapy
Endpoints of therapy
Indications
for treatment
Monitoring of patients currently not treated
Treatment strategies
Definition of response
to treatment
NA monotherapy
PegIFN monotherapy
Combination therapy
Patients with decompensated cirrhosis
Prevention of HBV recurrence after liver transplantation
Treatment in special patient groups
Click on a topic to skip to that section
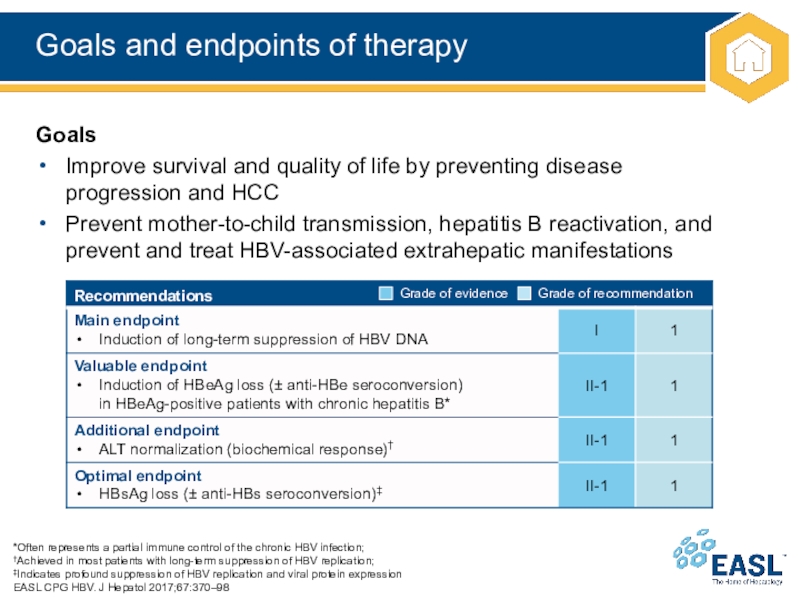
Слайд 14Goals and endpoints of therapy
*Often represents a partial immune control
of the chronic HBV infection;
†Achieved in most patients with long-term
suppression of HBV replication;
‡Indicates profound suppression of HBV replication and viral protein expression
EASL CPG HBV. J Hepatol 2017;67:370–98
Goals
Improve survival and quality of life by preventing disease
progression and HCC
Prevent mother-to-child transmission, hepatitis B reactivation, and prevent and treat HBV-associated extrahepatic manifestations
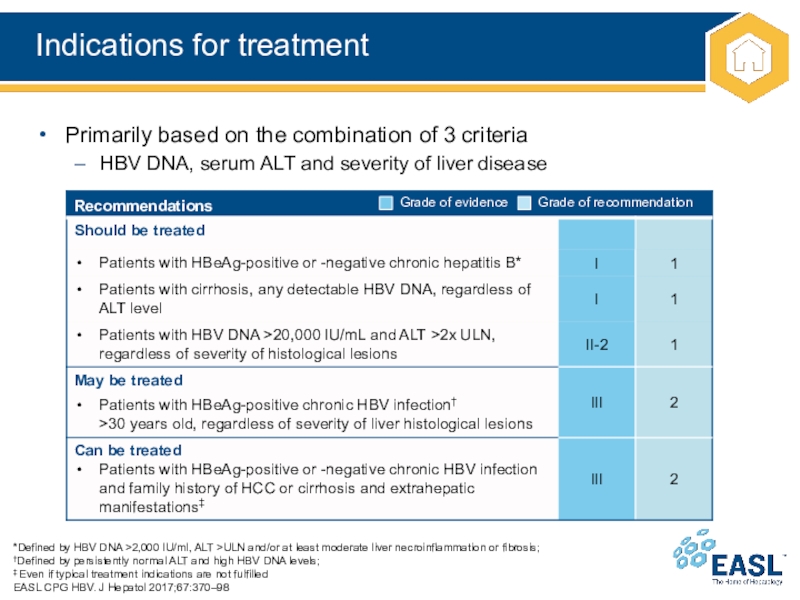
Слайд 15Indications for treatment
*Defined by HBV DNA >2,000 IU/ml, ALT >ULN
and/or at least moderate liver necroinflammation or fibrosis;
†Defined by persistently
normal ALT and high HBV DNA levels;
‡ Even if typical treatment indications are not fulfilled
EASL CPG HBV. J Hepatol 2017;67:370–98
Primarily based on the combination of 3 criteria
HBV DNA, serum ALT and severity of liver disease
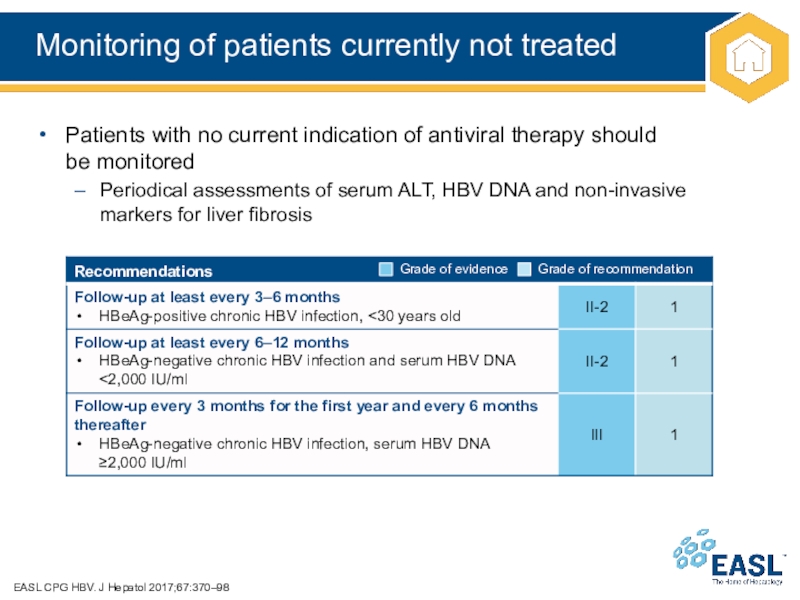
Слайд 16Monitoring of patients currently not treated
EASL CPG HBV. J Hepatol
2017;67:370–98
Patients with no current indication of antiviral therapy should
be
monitored
Periodical assessments of serum ALT, HBV DNA and non-invasive
markers for liver fibrosis
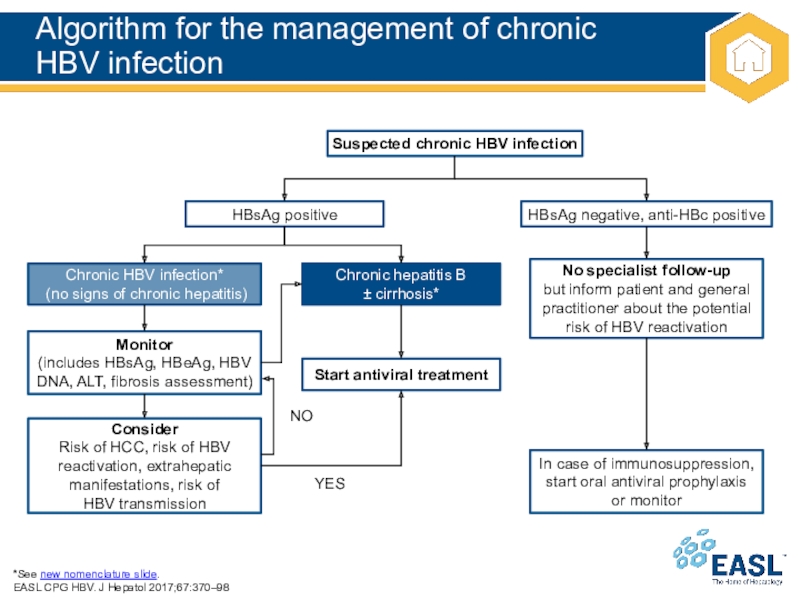
Слайд 17Algorithm for the management of chronic
HBV infection
*See new nomenclature
slide.
EASL CPG HBV. J Hepatol 2017;67:370–98
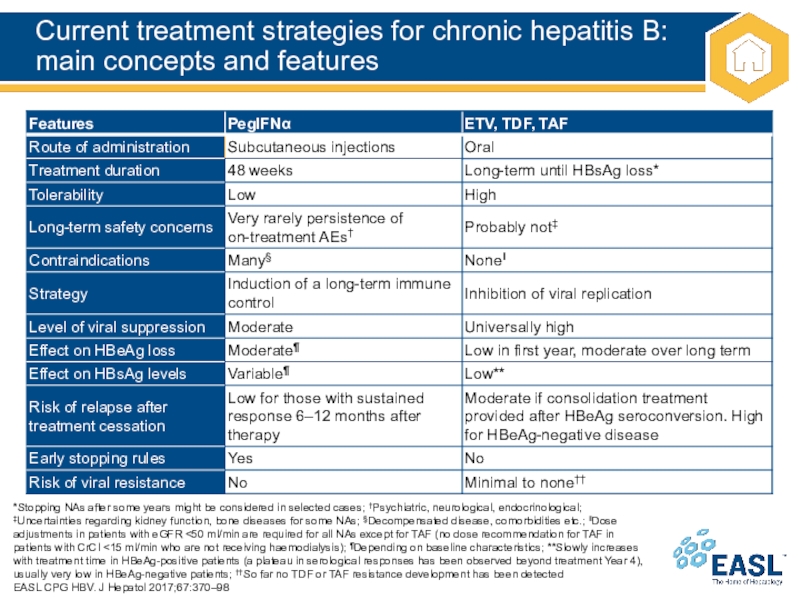
Слайд 18Current treatment strategies for chronic hepatitis B: main concepts and
features
*Stopping NAs after some years might be considered in selected
cases; †Psychiatric, neurological, endocrinological; ‡Uncertainties regarding kidney function, bone diseases for some NAs; §Decompensated disease, comorbidities etc.; ‖Dose adjustments in patients with eGFR <50 ml/min are required for all NAs except for TAF (no dose recommendation for TAF in patients with CrCl <15 ml/min who are not receiving haemodialysis); ¶Depending on baseline characteristics; **Slowly increases with treatment time in HBeAg-positive patients (a plateau in serological responses has been observed beyond treatment Year 4), usually very low in HBeAg-negative patients; ††So far no TDF or TAF resistance development has been detected
EASL CPG HBV. J Hepatol 2017;67:370–98
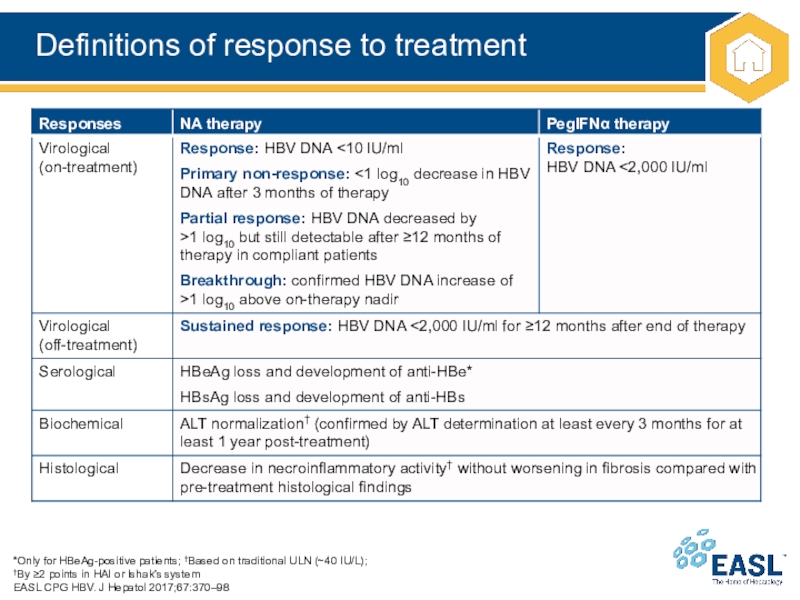
Слайд 19Definitions of response to treatment
*Only for HBeAg-positive patients; †Based on
traditional ULN (~40 IU/L);
†By ≥2 points in HAI or Ishak’s
system
EASL CPG HBV. J Hepatol 2017;67:370–98
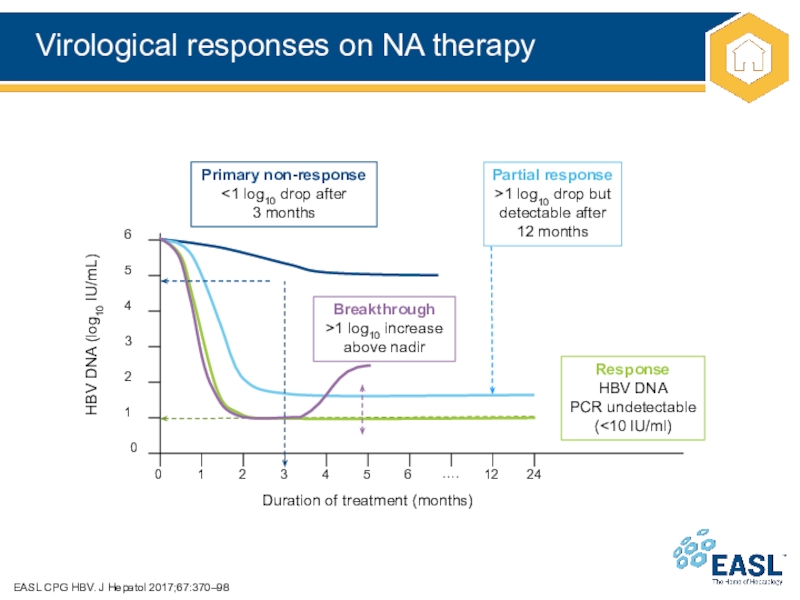
Слайд 20Virological responses on NA therapy
EASL CPG HBV. J Hepatol 2017;67:370–98
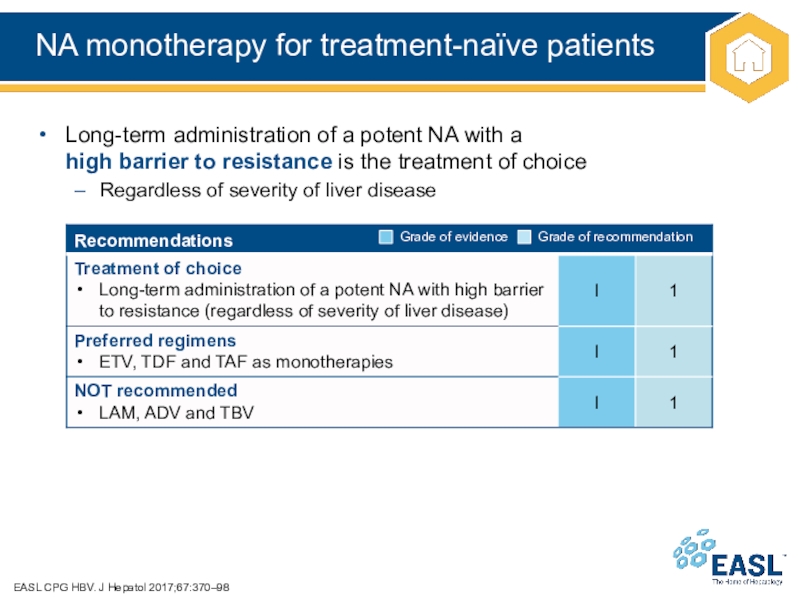
Слайд 21NA monotherapy for treatment-naïve patients
EASL CPG HBV. J Hepatol 2017;67:370–98
Long-term
administration of a potent NA with a
high barrier to
resistance is the treatment of choice
Regardless of severity of liver disease
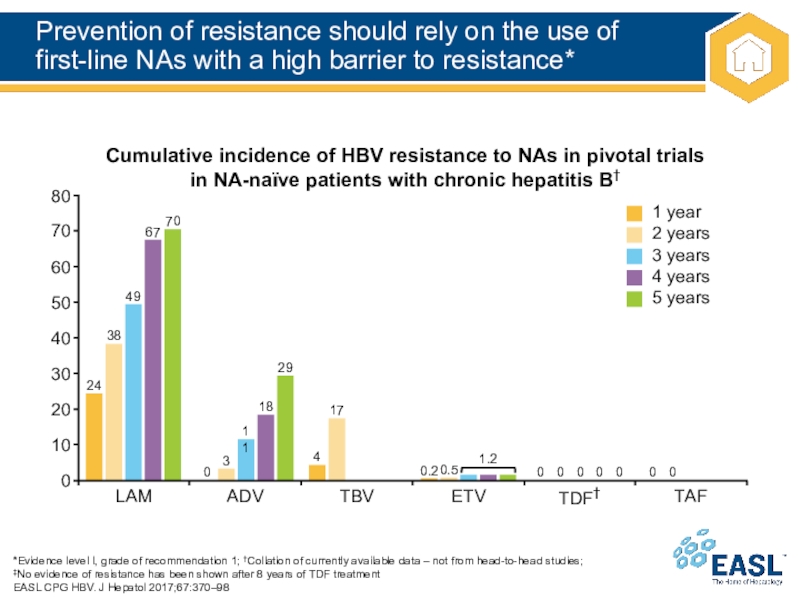
Слайд 22Prevention of resistance should rely on the use of
first-line NAs
with a high barrier to resistance*
*Evidence level I, grade of
recommendation 1; †Collation of currently available data – not from head-to-head studies;
‡No evidence of resistance has been shown after 8 years of TDF treatment
EASL CPG HBV. J Hepatol 2017;67:370–98
Cumulative incidence of HBV resistance to NAs in pivotal trials in NA-naïve patients with chronic hepatitis B†
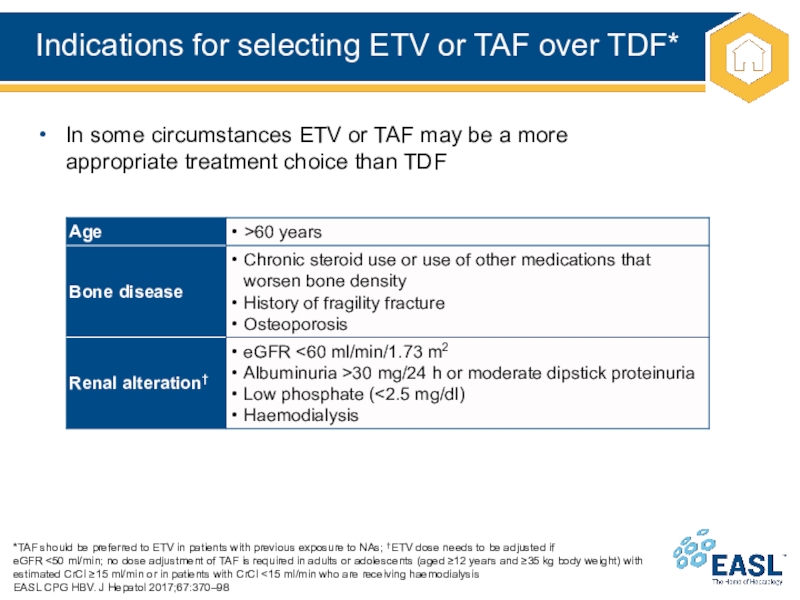
Слайд 23Indications for selecting ETV or TAF over TDF*
*TAF should be
preferred to ETV in patients with previous exposure to NAs;
†ETV dose needs to be adjusted if
eGFR <50 ml/min; no dose adjustment of TAF is required in adults or adolescents (aged ≥12 years and ≥35 kg body weight) with estimated CrCl ≥15 ml/min or in patients with CrCl <15 ml/min who are receiving haemodialysis
EASL CPG HBV. J Hepatol 2017;67:370–98
In some circumstances ETV or TAF may be a more
appropriate treatment choice than TDF
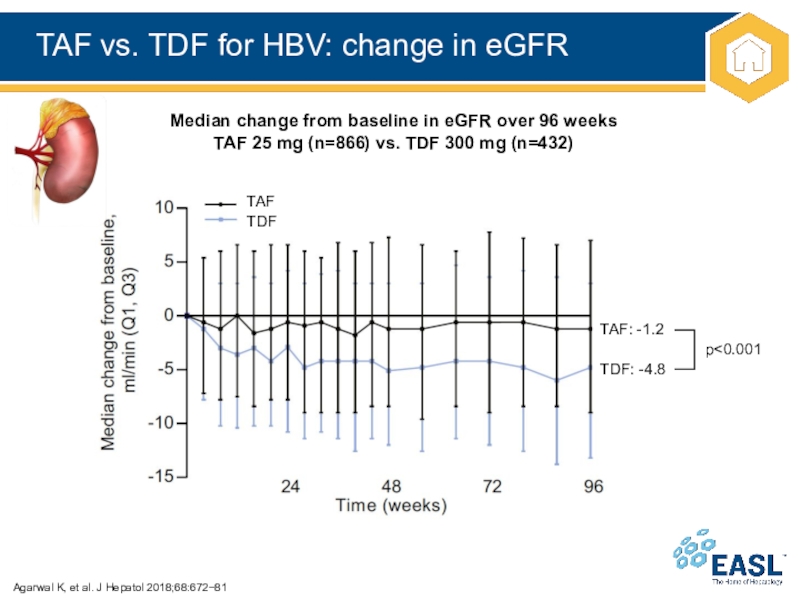
Слайд 24TAF vs. TDF for HBV: change in eGFR
Agarwal K, et
al. J Hepatol 2018;68:67281
Median change from baseline in eGFR over
96 weeks
TAF 25 mg (n=866) vs. TDF 300 mg (n=432)
TAF
TDF
TAF: -1.2
TDF: -4.8
p<0.001
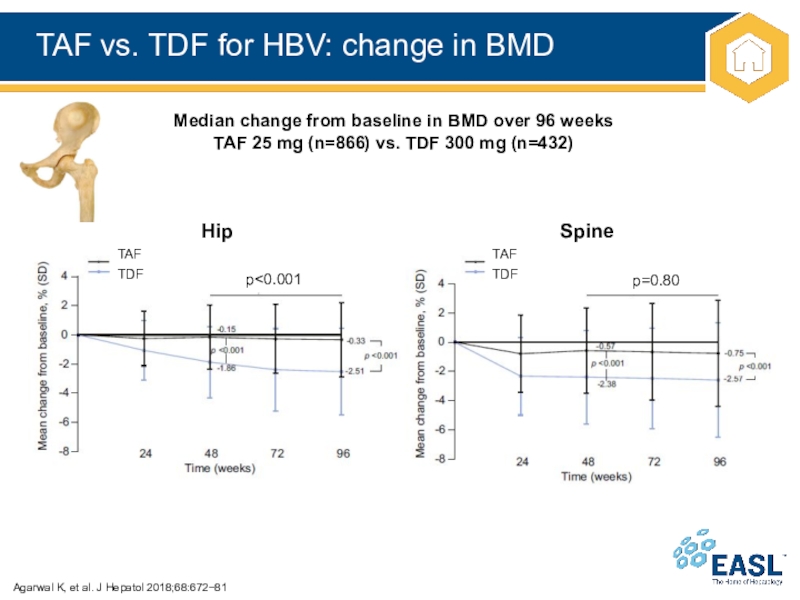
Слайд 25TAF vs. TDF for HBV: change in BMD
Agarwal K, et
al. J Hepatol 2018;68:67281
Median change from baseline in BMD over
96 weeks
TAF 25 mg (n=866) vs. TDF 300 mg (n=432)
TAF
TDF
Hip
Spine
TAF
TDF
p<0.001
p=0.80
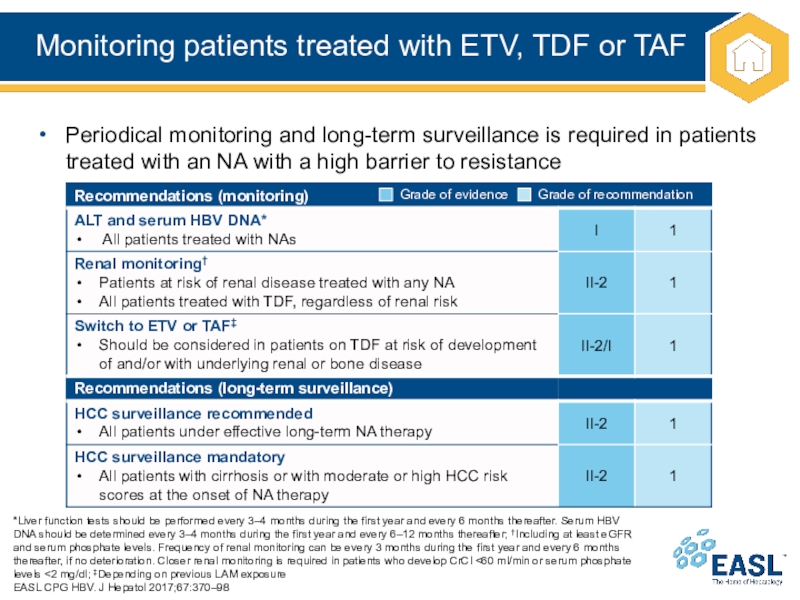
Слайд 26Monitoring patients treated with ETV, TDF or TAF
*Liver function tests
should be performed every 3–4 months during the first year
and every 6 months thereafter. Serum HBV DNA should be determined every 3–4 months during the first year and every 6–12 months thereafter; †Including at least eGFR and serum phosphate levels. Frequency of renal monitoring can be every 3 months during the first year and every 6 months thereafter, if no deterioration. Closer renal monitoring is required in patients who develop CrCl <60 ml/min or serum phosphate levels <2 mg/dl; ‡Depending on previous LAM exposure
EASL CPG HBV. J Hepatol 2017;67:370–98
Periodical monitoring and long-term surveillance is required in patients treated with an NA with a high barrier to resistance
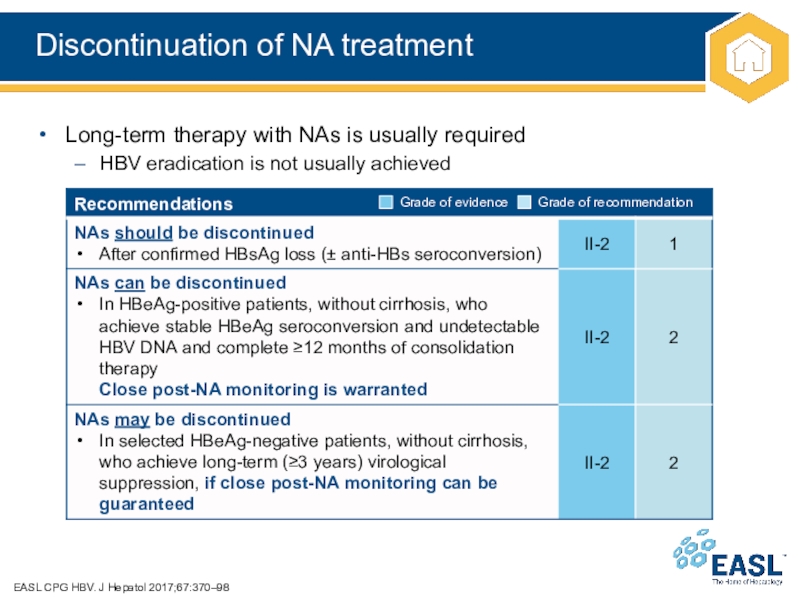
Слайд 27Discontinuation of NA treatment
EASL CPG HBV. J Hepatol 2017;67:370–98
Long-term therapy
with NAs is usually required
HBV eradication is not usually achieved
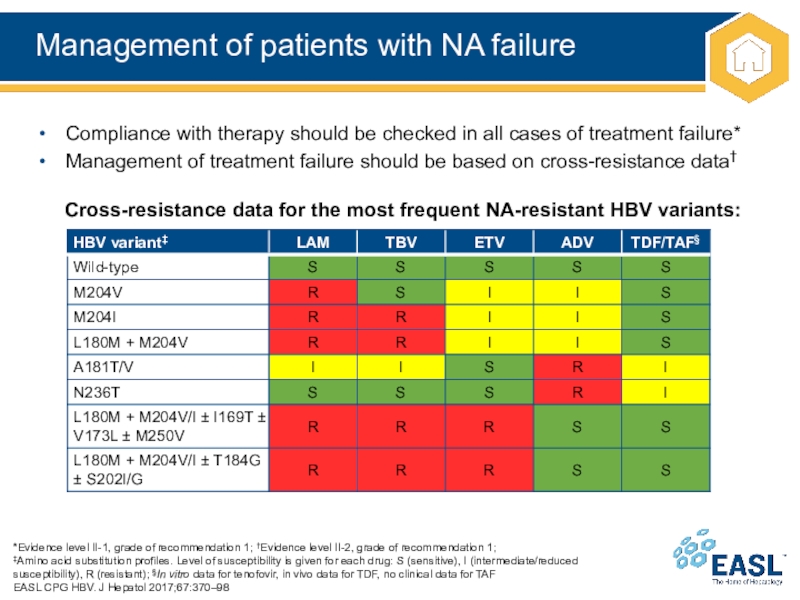
Слайд 28Management of patients with NA failure
*Evidence level II-1, grade of
recommendation 1; †Evidence level II-2, grade of recommendation 1;
‡Amino
acid substitution profiles. Level of susceptibility is given for each drug: S (sensitive), I (intermediate/reduced susceptibility), R (resistant); §In vitro data for tenofovir, in vivo data for TDF, no clinical data for TAF
EASL CPG HBV. J Hepatol 2017;67:370–98
Compliance with therapy should be checked in all cases of treatment failure*
Management of treatment failure should be based on cross-resistance data†
Cross-resistance data for the most frequent NA-resistant HBV variants:
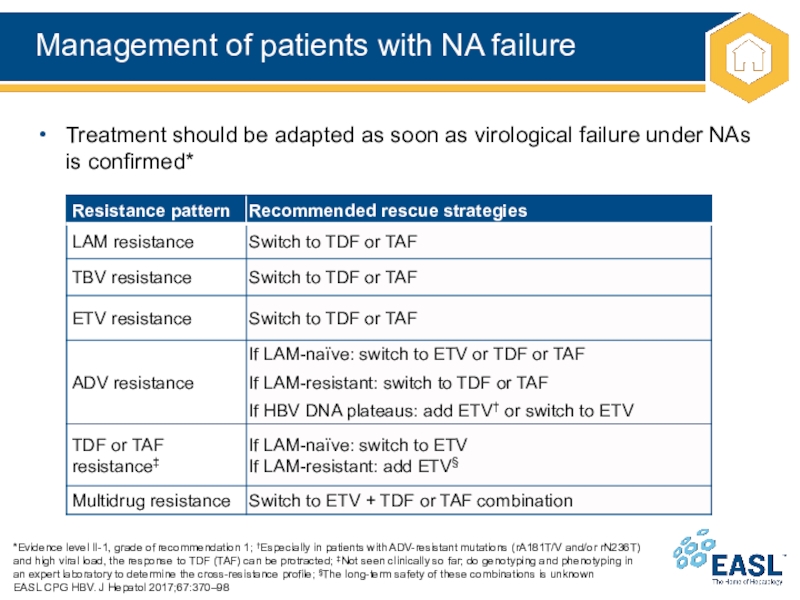
Слайд 29Management of patients with NA failure
*Evidence level II-1, grade of
recommendation 1; †Especially in patients with ADV-resistant mutations (rA181T/V and/or
rN236T) and high viral load, the response to TDF (TAF) can be protracted; ‡Not seen clinically so far; do genotyping and phenotyping in an expert laboratory to determine the cross-resistance profile; §The long-term safety of these combinations is unknown
EASL CPG HBV. J Hepatol 2017;67:370–98
Treatment should be adapted as soon as virological failure under NAs is confirmed*
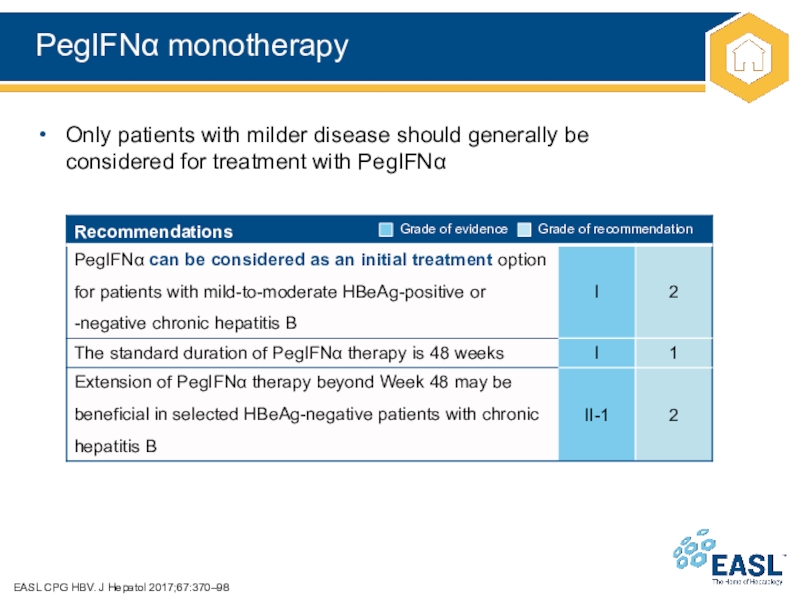
Слайд 30PegIFN monotherapy
EASL CPG HBV. J Hepatol 2017;67:370–98
Only patients with milder
disease should generally be
considered for treatment with PegIFN
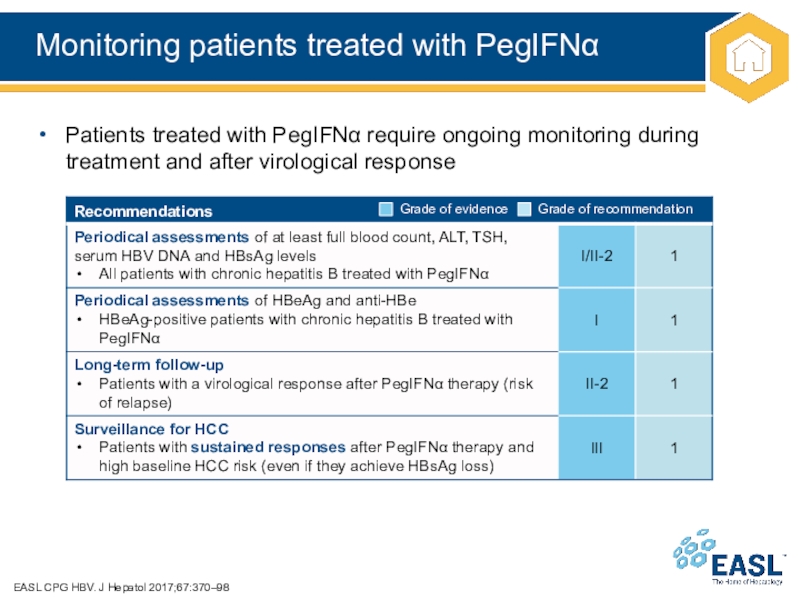
Слайд 31Monitoring patients treated with PegIFN
EASL CPG HBV. J Hepatol 2017;67:370–98
Patients
treated with PegIFN require ongoing monitoring during treatment and after
virological response
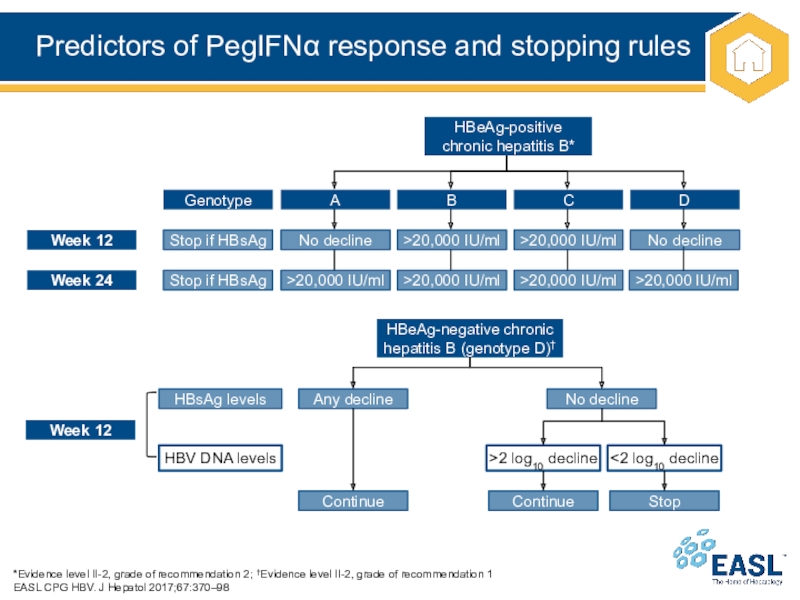
Слайд 32Predictors of PegIFN response and stopping rules
*Evidence level II-2, grade
of recommendation 2; †Evidence level II-2, grade of recommendation 1
EASL
CPG HBV. J Hepatol 2017;67:370–98
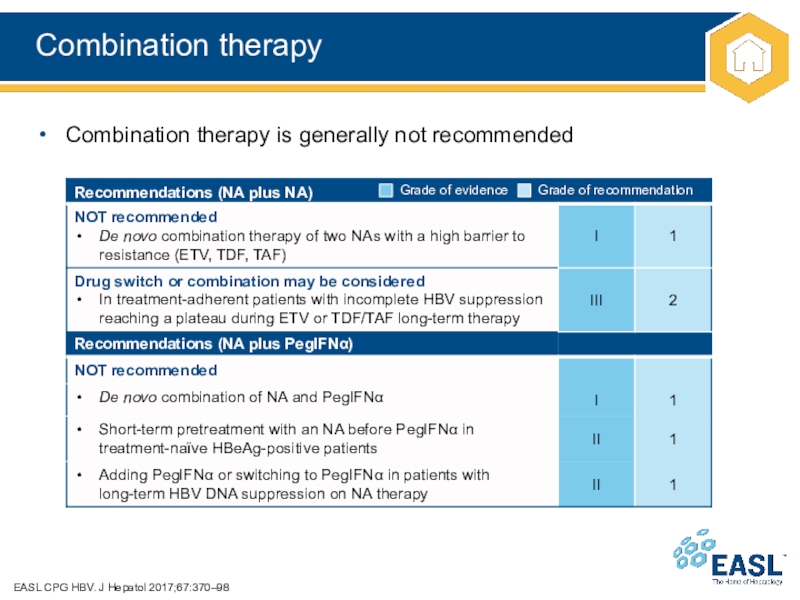
Слайд 33Combination therapy
EASL CPG HBV. J Hepatol 2017;67:370–98
Combination therapy is generally
not recommended
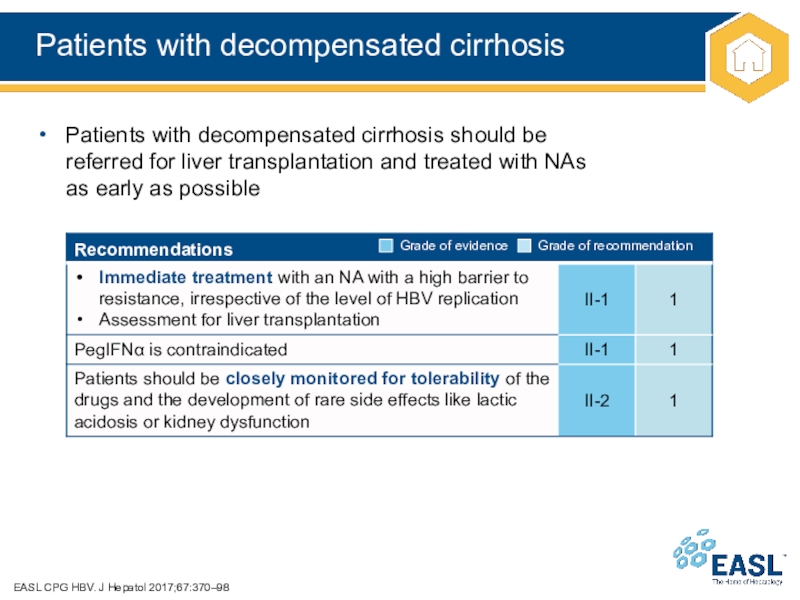
Слайд 34Patients with decompensated cirrhosis
EASL CPG HBV. J Hepatol 2017;67:370–98
Patients with
decompensated cirrhosis should be
referred for liver transplantation and treated with
NAs
as early as possible
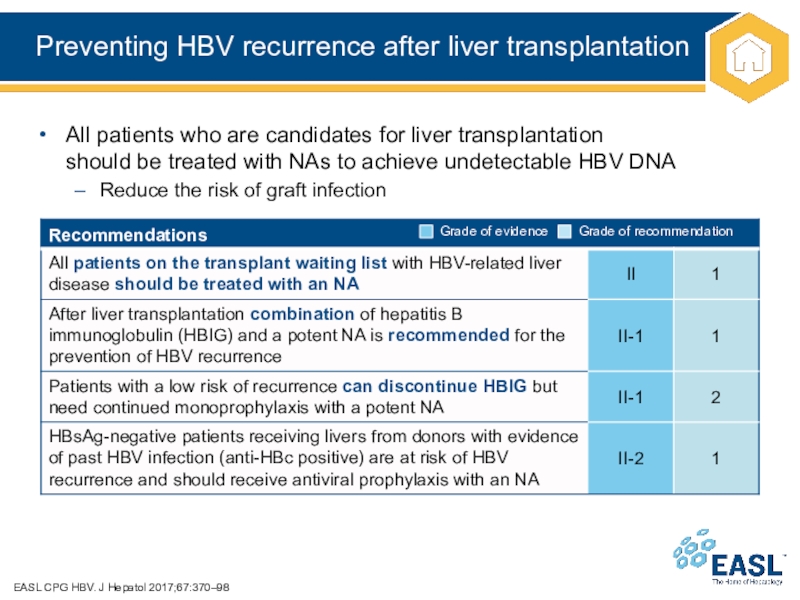
Слайд 35Preventing HBV recurrence after liver transplantation
EASL CPG HBV. J Hepatol
2017;67:370–98
All patients who are candidates for liver transplantation
should be treated
with NAs to achieve undetectable HBV DNA
Reduce the risk of graft infection
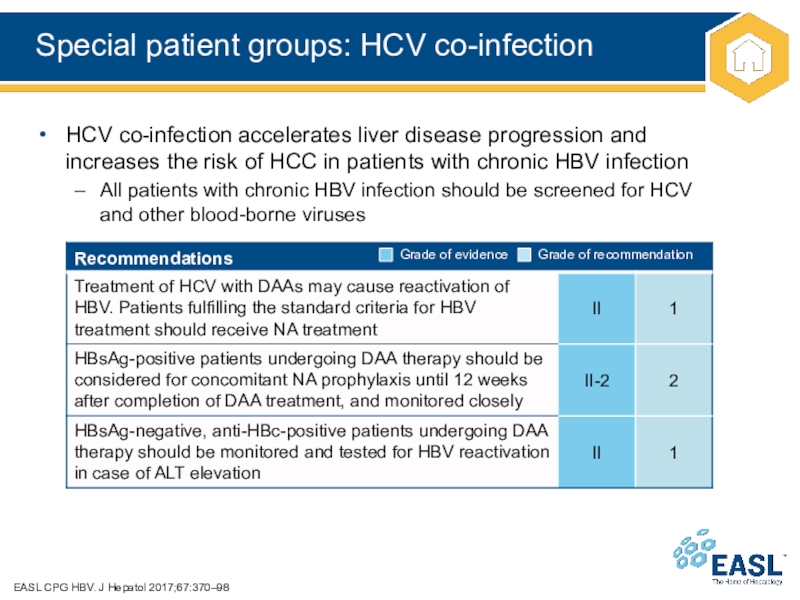
Слайд 36Special patient groups: HCV co-infection
EASL CPG HBV. J Hepatol
2017;67:370–98
HCV co-infection accelerates liver disease progression and
increases the risk of
HCC in patients with chronic HBV infection
All patients with chronic HBV infection should be screened for HCV
and other blood-borne viruses
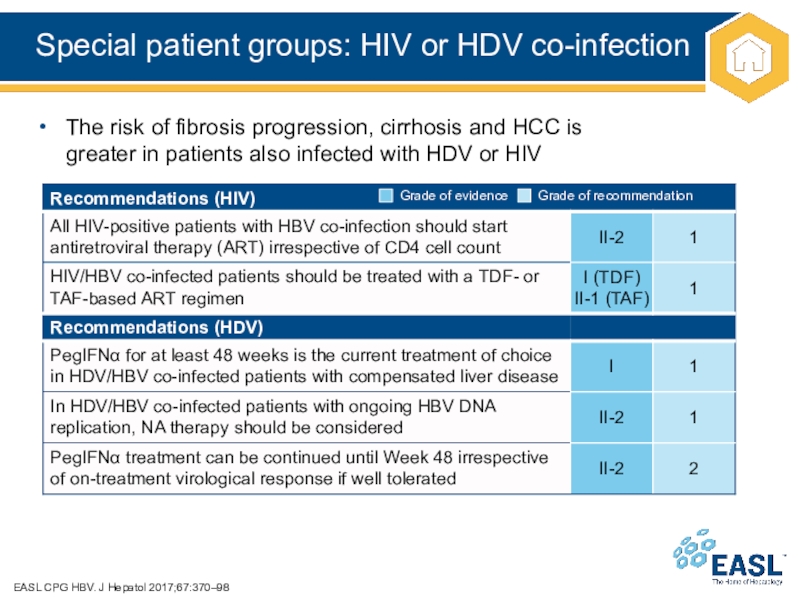
Слайд 37Special patient groups: HIV or HDV co-infection
EASL CPG HBV.
J Hepatol 2017;67:370–98
The risk of fibrosis progression, cirrhosis and HCC
is
greater in patients also infected with HDV or HIV
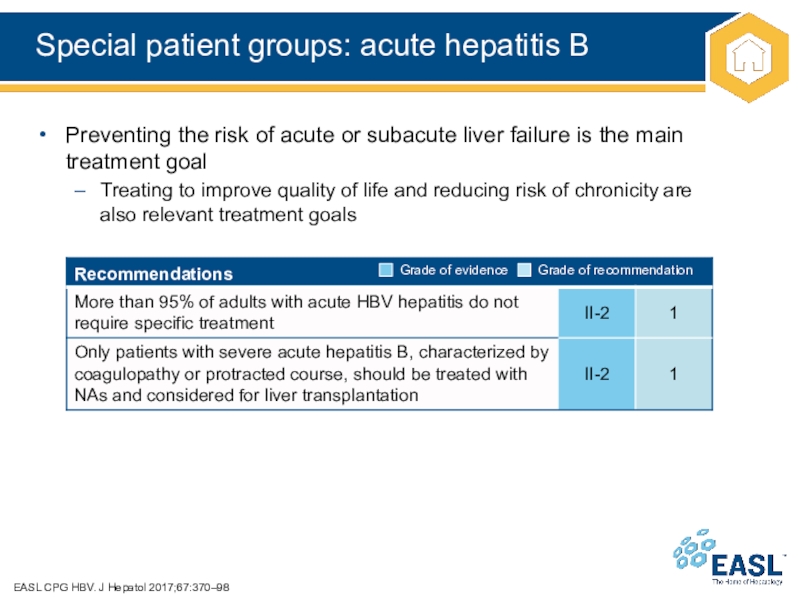
Слайд 38Special patient groups: acute hepatitis B
EASL CPG HBV. J Hepatol
2017;67:370–98
Preventing the risk of acute or subacute liver failure is
the main treatment goal
Treating to improve quality of life and reducing risk of chronicity are
also relevant treatment goals
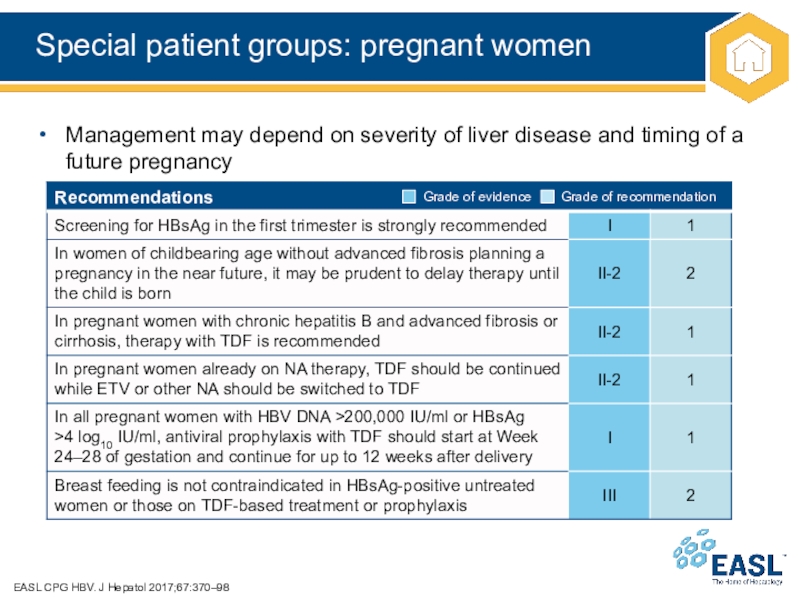
Слайд 39Special patient groups: pregnant women
EASL CPG HBV. J Hepatol 2017;67:370–98
Management
may depend on severity of liver disease and timing of
a future pregnancy
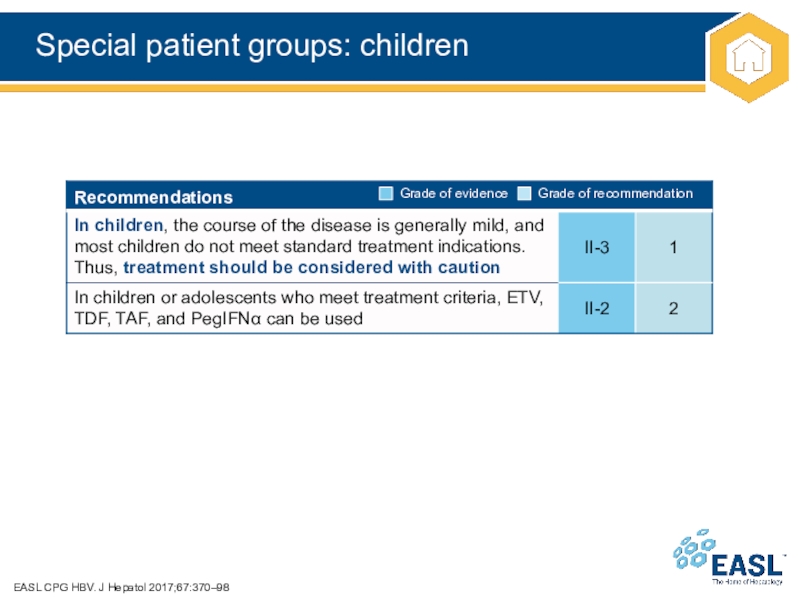
Слайд 40Special patient groups: children
EASL CPG HBV. J Hepatol 2017;67:370–98
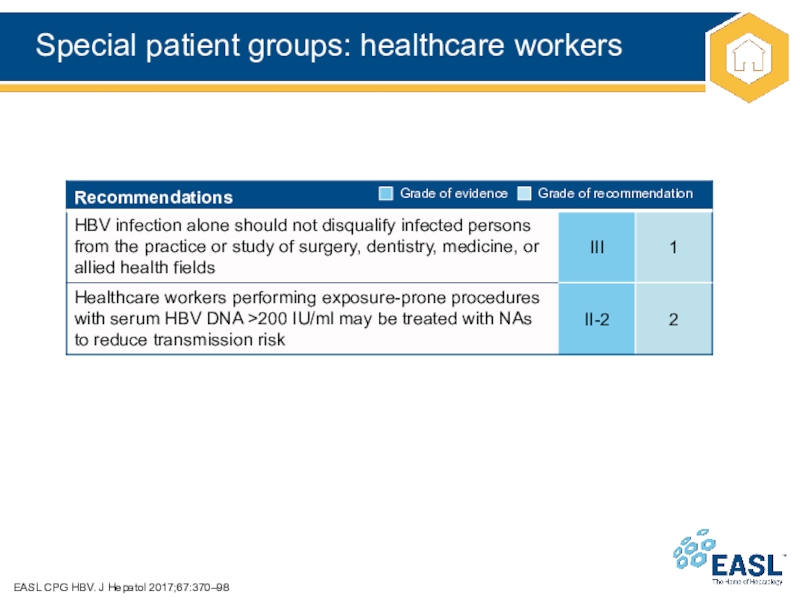
Слайд 41Special patient groups: healthcare workers
EASL CPG HBV. J Hepatol 2017;67:370–98
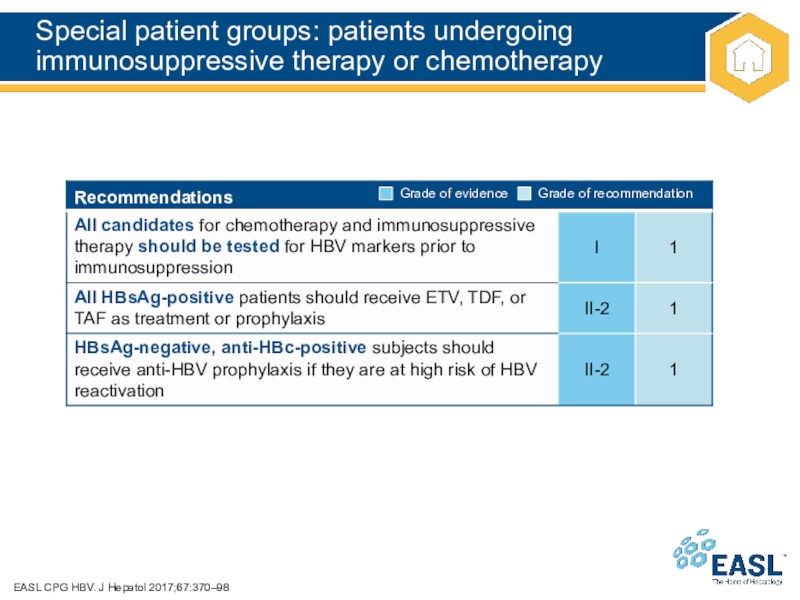
Слайд 42Special patient groups: patients undergoing immunosuppressive therapy or chemotherapy
EASL CPG
HBV. J Hepatol 2017;67:370–98
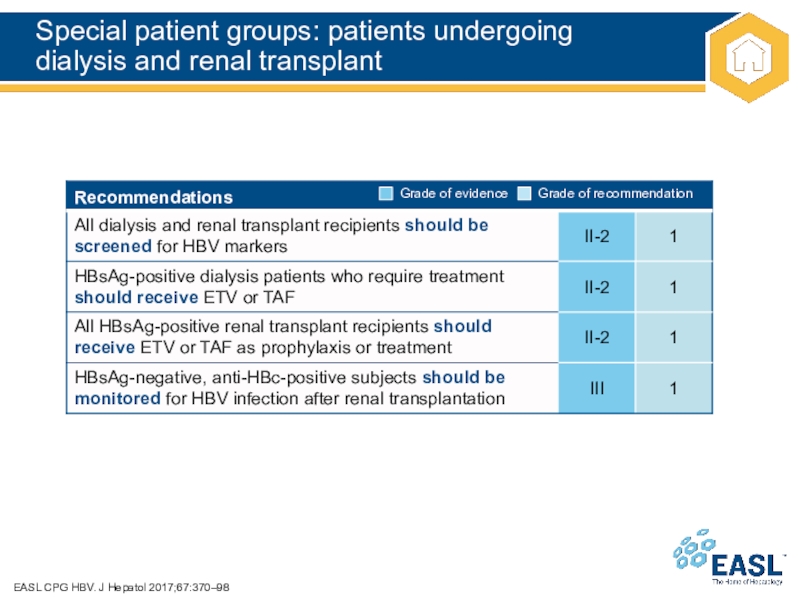
Слайд 43Special patient groups: patients undergoing
dialysis and renal transplant
EASL CPG
HBV. J Hepatol 2017;67:370–98
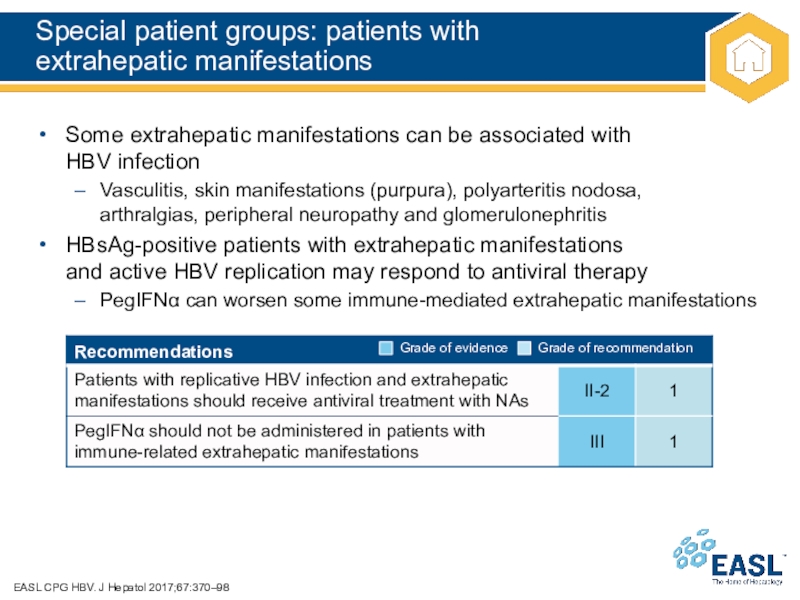
Слайд 44Special patient groups: patients with
extrahepatic manifestations
EASL CPG HBV. J
Hepatol 2017;67:370–98
Some extrahepatic manifestations can be associated with
HBV infection
Vasculitis,
skin manifestations (purpura), polyarteritis nodosa,
arthralgias, peripheral neuropathy and glomerulonephritis
HBsAg-positive patients with extrahepatic manifestations
and active HBV replication may respond to antiviral therapy
PegIFN can worsen some immune-mediated extrahepatic manifestations
Слайд 45The future for HBV
New biomarkers
Future treatments
Unresolved issues
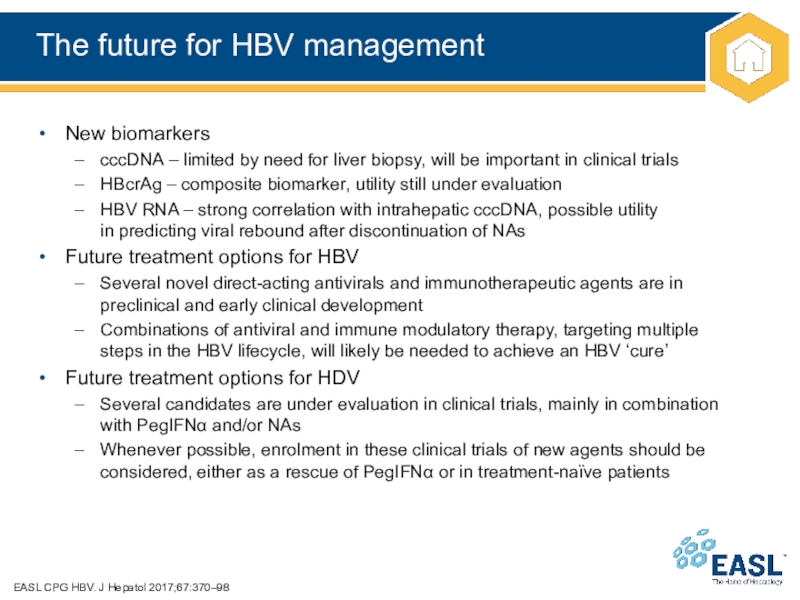
Слайд 46The future for HBV management
EASL CPG HBV. J Hepatol 2017;67:370–98
New
biomarkers
cccDNA – limited by need for liver biopsy, will
be important in clinical trials
HBcrAg – composite biomarker, utility still under evaluation
HBV RNA – strong correlation with intrahepatic cccDNA, possible utility
in predicting viral rebound after discontinuation of NAs
Future treatment options for HBV
Several novel direct-acting antivirals and immunotherapeutic agents are in preclinical and early clinical development
Combinations of antiviral and immune modulatory therapy, targeting multiple
steps in the HBV lifecycle, will likely be needed to achieve an HBV ‘cure’
Future treatment options for HDV
Several candidates are under evaluation in clinical trials, mainly in combination
with PegIFN and/or NAs
Whenever possible, enrolment in these clinical trials of new agents should be considered, either as a rescue of PegIFN or in treatment-naïve patients
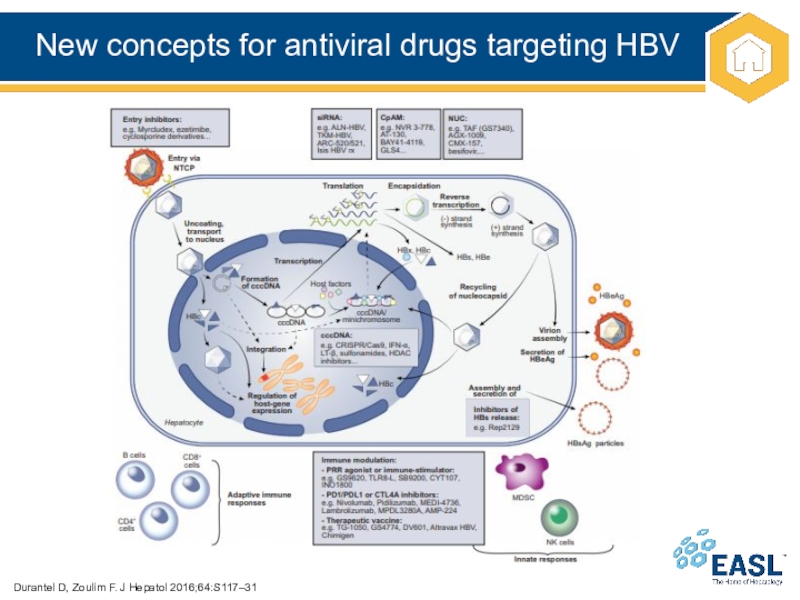
Слайд 47New concepts for antiviral drugs targeting HBV
Durantel D, Zoulim F.
J Hepatol 2016;64:S117–31
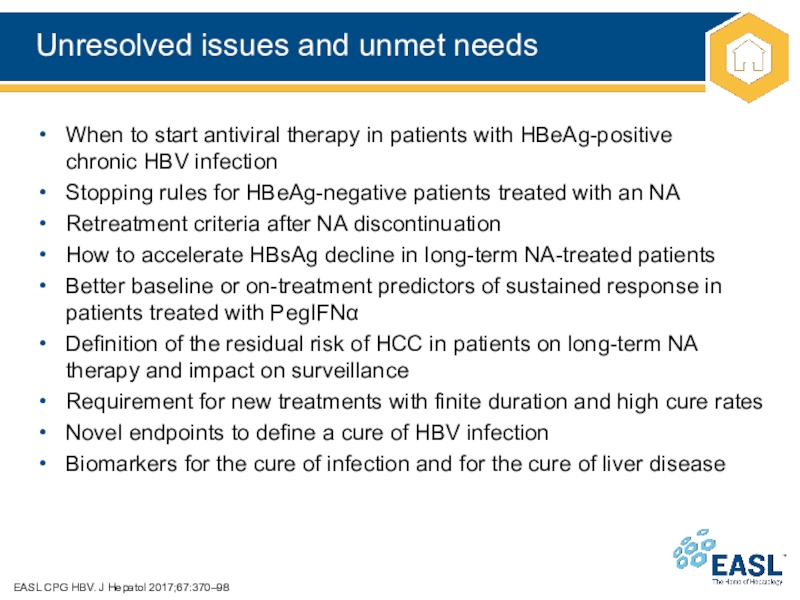
Слайд 48Unresolved issues and unmet needs
EASL CPG HBV. J Hepatol 2017;67:370–98
When
to start antiviral therapy in patients with HBeAg-positive
chronic HBV infection
Stopping
rules for HBeAg-negative patients treated with an NA
Retreatment criteria after NA discontinuation
How to accelerate HBsAg decline in long-term NA-treated patients
Better baseline or on-treatment predictors of sustained response in patients treated with PegIFN
Definition of the residual risk of HCC in patients on long-term NA therapy and impact on surveillance
Requirement for new treatments with finite duration and high cure rates
Novel endpoints to define a cure of HBV infection
Biomarkers for the cure of infection and for the cure of liver disease