Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
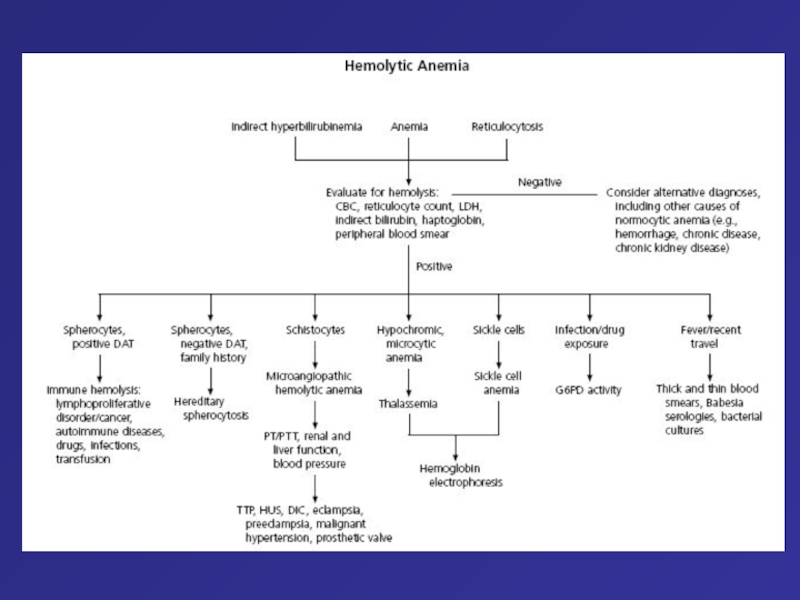
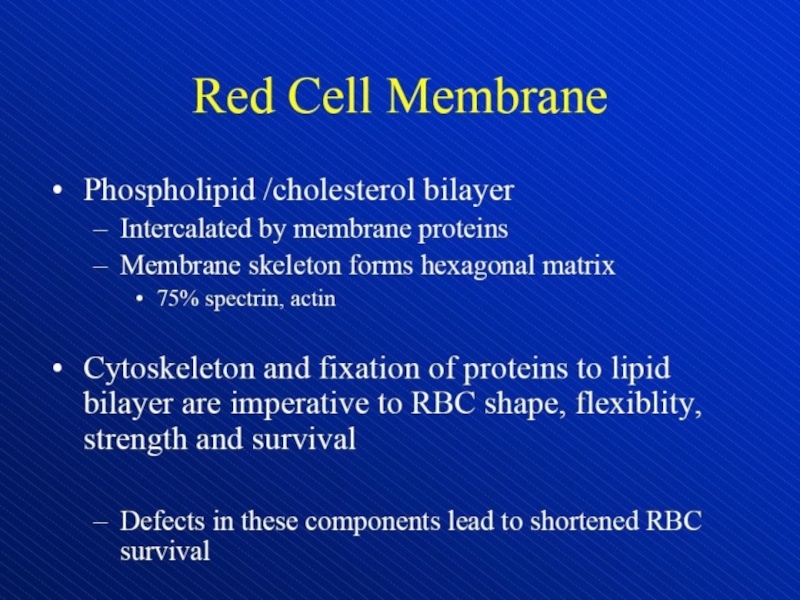
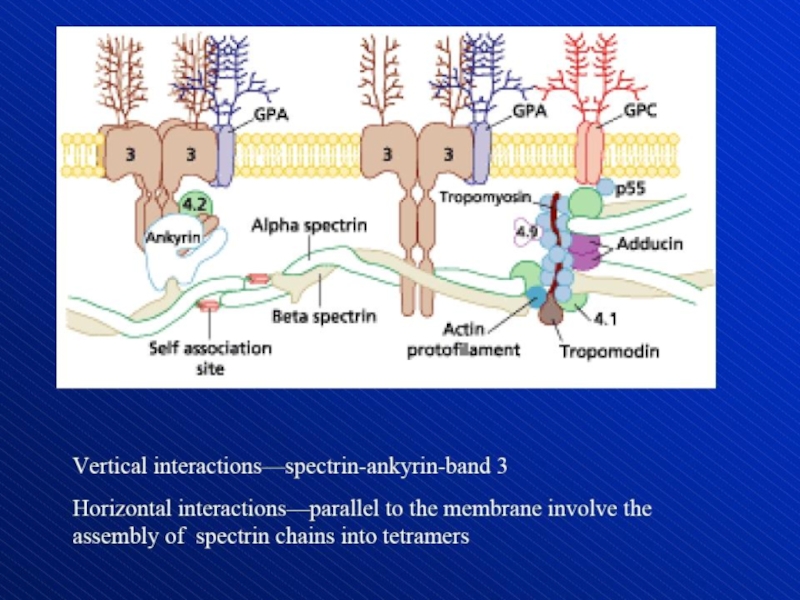
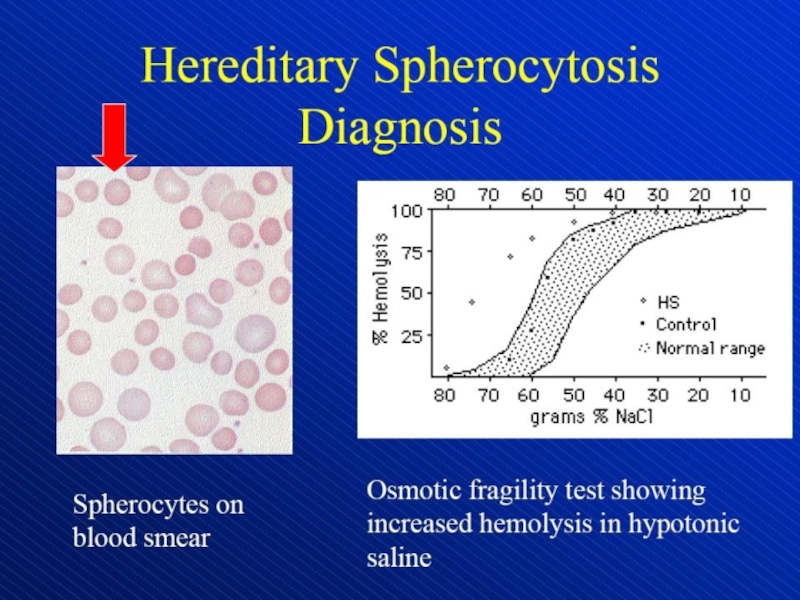
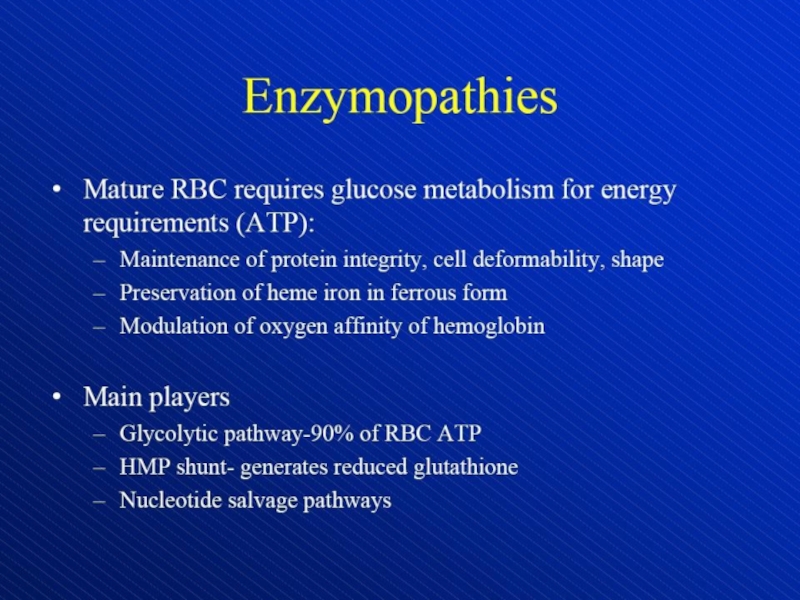
Hemolytic anemia – increased destruction of erythrocytes
Содержание
- 1. Hemolytic anemia – increased destruction of erythrocytes
- 2. Hemolytic anemia – increased destruction of erythrocyteswith adequate response of bone marrow.
- 3. Слайд 3
- 4. Слайд 4
- 5. Слайд 5
- 6. Слайд 6
- 7. Слайд 7
- 8. Слайд 8
- 9. Слайд 9
- 10. Слайд 10
- 11. Слайд 11
- 12. Слайд 12
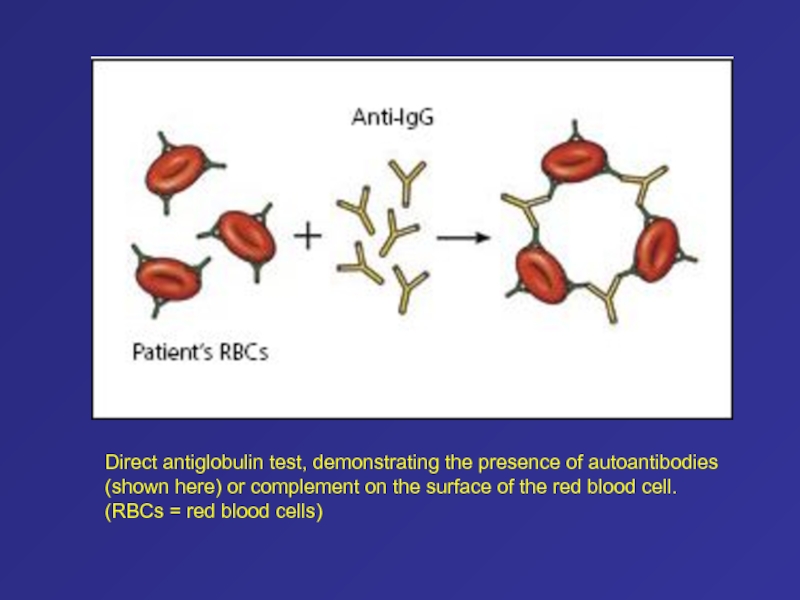
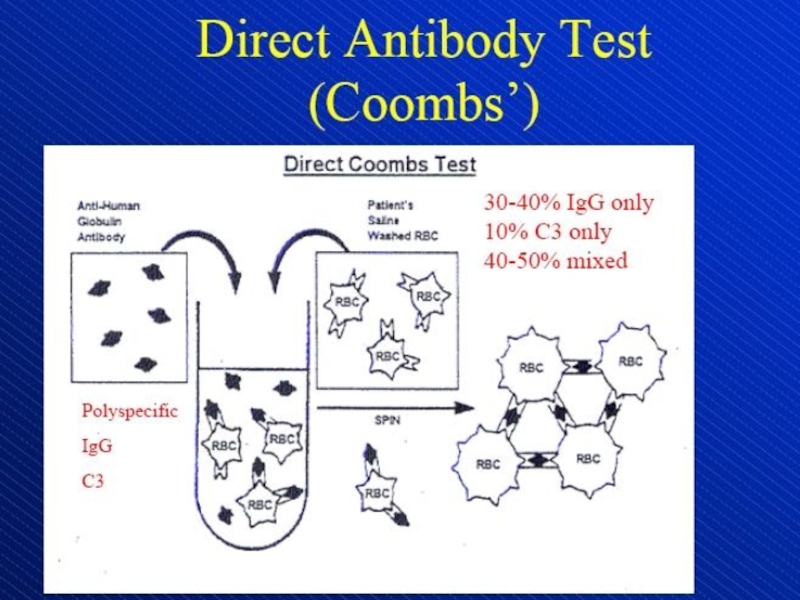
- 13. Direct antiglobulin test, demonstrating the presence of
- 14. Слайд 14
- 15. Слайд 15
- 16. Слайд 16
- 17. Слайд 17
- 18. Слайд 18
- 19. Слайд 19
- 20. Слайд 20
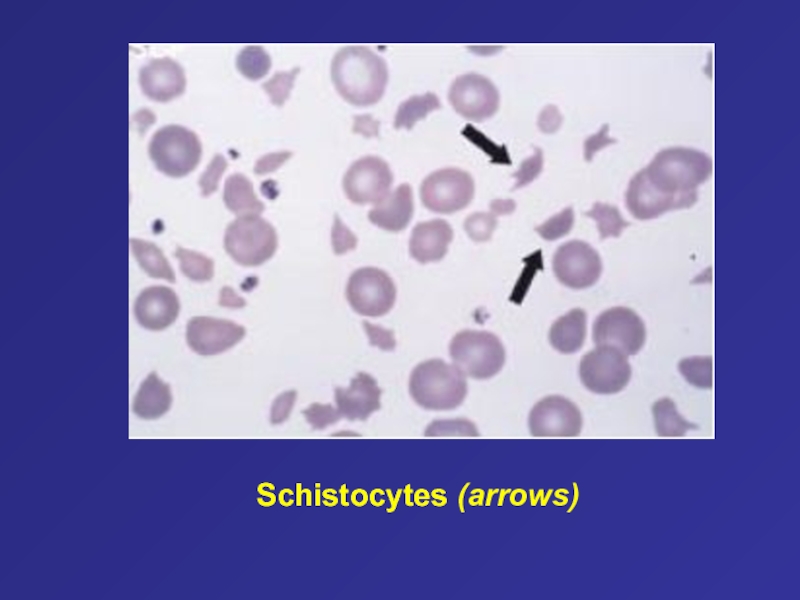
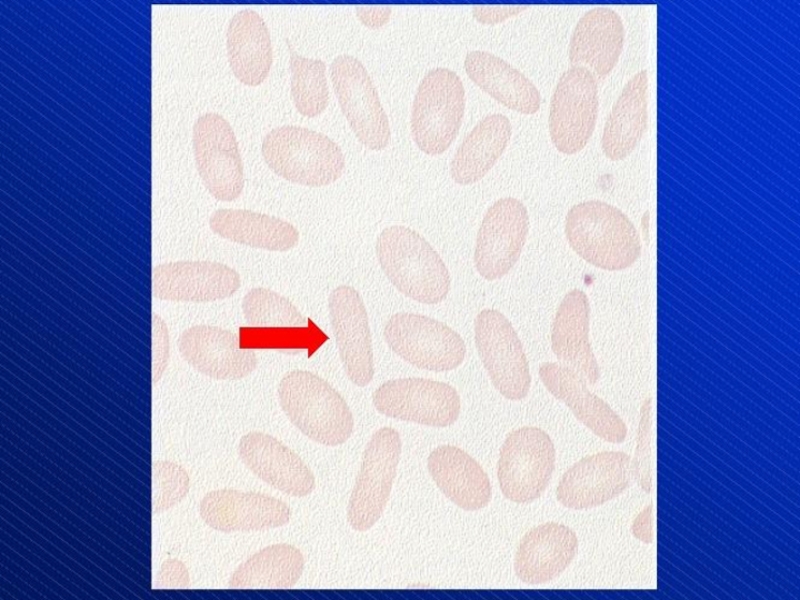
- 21. Schistocytes (arrows)
- 22. Слайд 22
- 23. Слайд 23
- 24. Слайд 24
- 25. Слайд 25
- 26. Слайд 26
- 27. Слайд 27
- 28. Слайд 28
- 29. Слайд 29
- 30. Слайд 30
- 31. Слайд 31
- 32. Слайд 32
- 33. Слайд 33
- 34. Слайд 34
- 35. Слайд 35
- 36. Слайд 36
- 37. Слайд 37
- 38. Слайд 38
- 39. Слайд 39
- 40. Слайд 40
- 41. Слайд 41
- 42. Слайд 42
- 43. Слайд 43
- 44. Слайд 44
- 45. Слайд 45
- 46. Слайд 46
- 47. Слайд 47
- 48. Слайд 48
- 49. Слайд 49
- 50. Слайд 50
- 51. Слайд 51
- 52. PNHMutations – Chromosome X (PIG-Agene) Deletions Insertion Point mutationsLoss FunctionLoss
- 53. SleepingHypoventilationAcidofication of PlasmaComplement + ActivationIncreased HemolysisPNH
- 54. Increased soluble uPARVenous thrombotic eventsDeficiency of the receptor for urokinase type plasminogen activator (uPAR)
- 55. Слайд 55
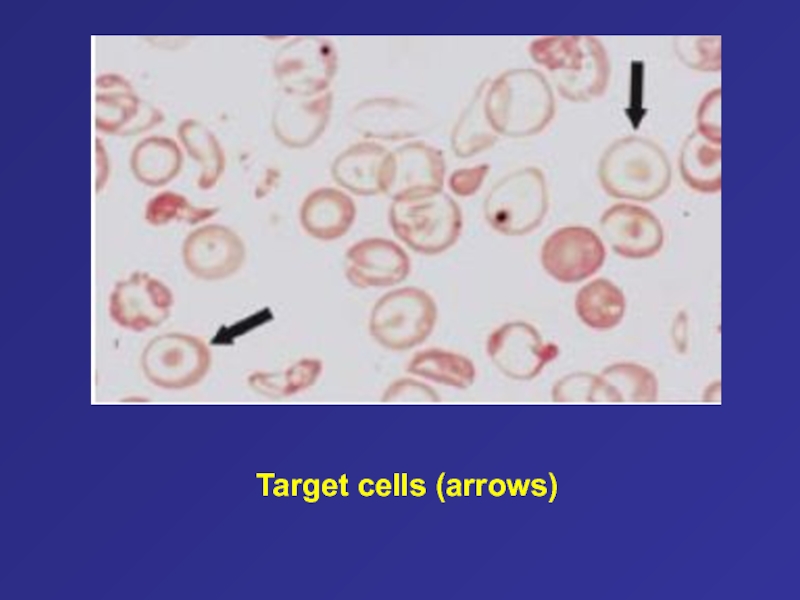
- 56. Target cells (arrows)
- 57. Three-step model for the development of paroxysmal
- 58. Relationship between bone marrow failure syndromes, including
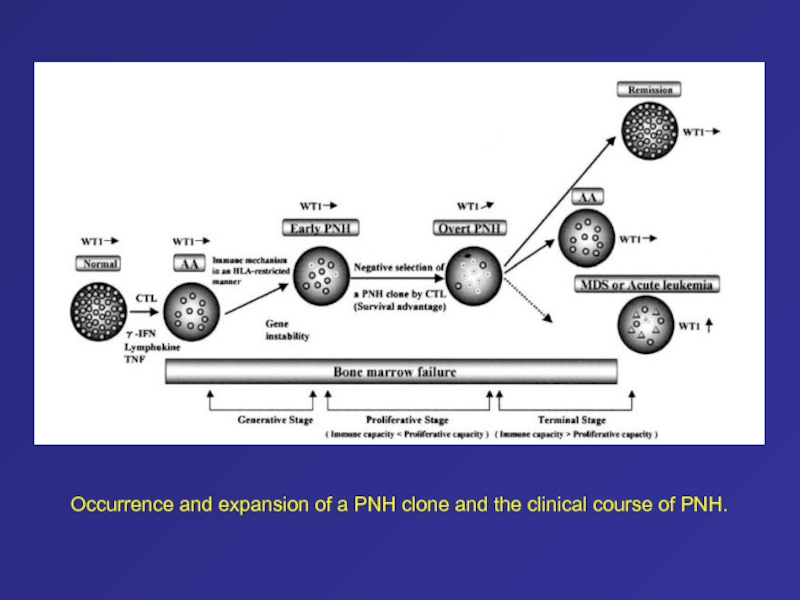
- 59. Occurrence and expansion of a PNH clone and the clinical course of PNH.
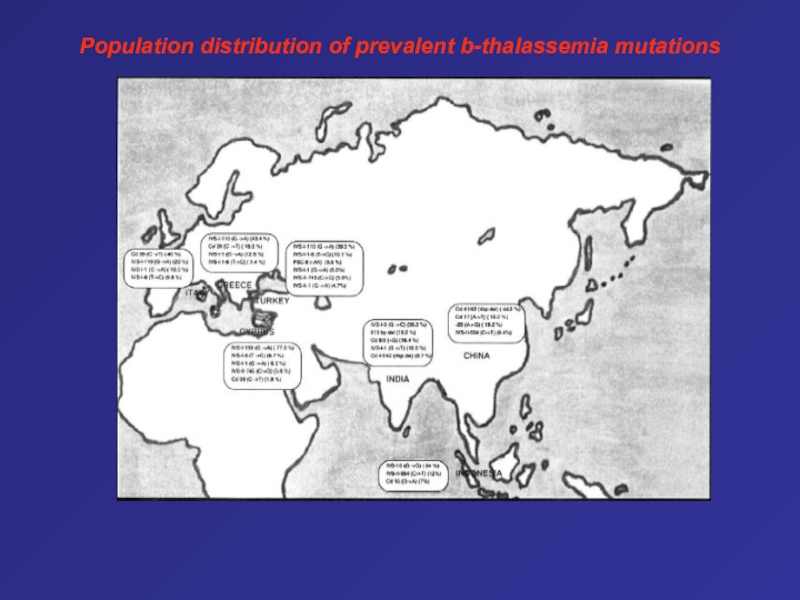
- 60. Population distribution of prevalent b-thalassemia mutations
- 61. Early in fetal life the synthesis of
- 62. Types of Hemoglobinα2γ2
- 63. The ß-Globin Gene Cluster on the Short
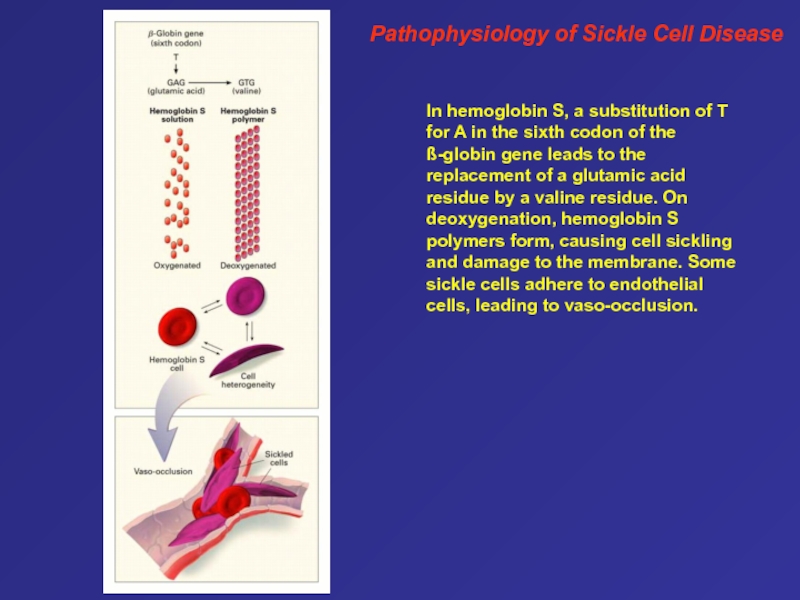
- 64. Pathophysiology of Sickle Cell Disease In hemoglobin
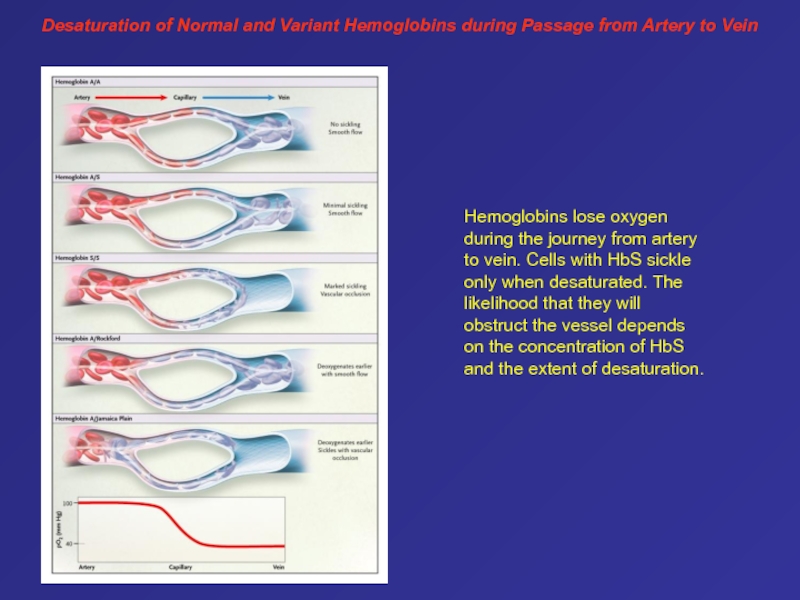
- 65. Desaturation of Normal and Variant Hemoglobins during
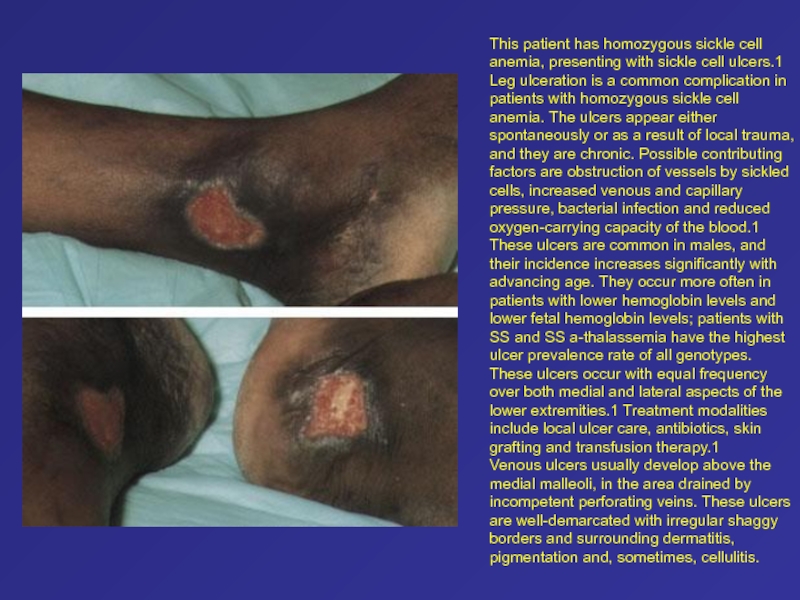
- 66. This patient has homozygous sickle cell anemia,
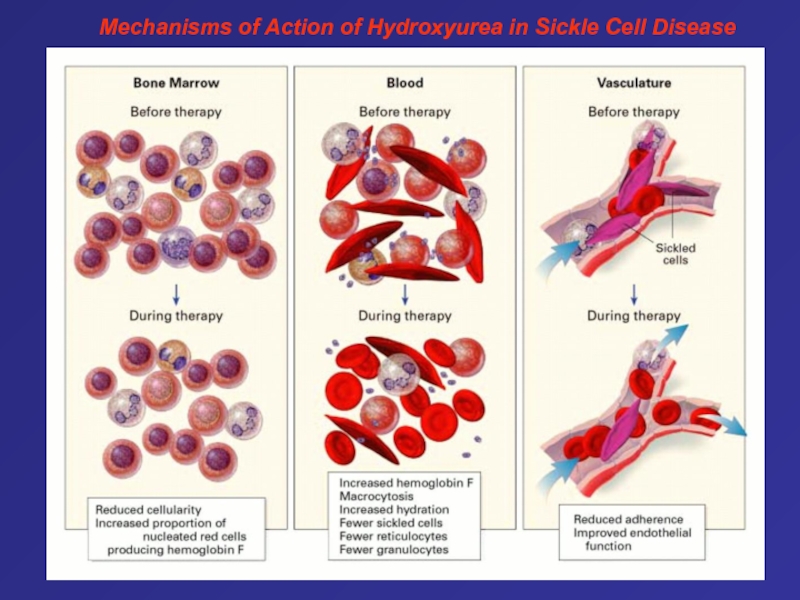
- 67. Mechanisms of Action of Hydroxyurea in Sickle Cell Disease
- 68. Слайд 68
- 69. Слайд 69
- 70. Слайд 70
- 71. Слайд 71
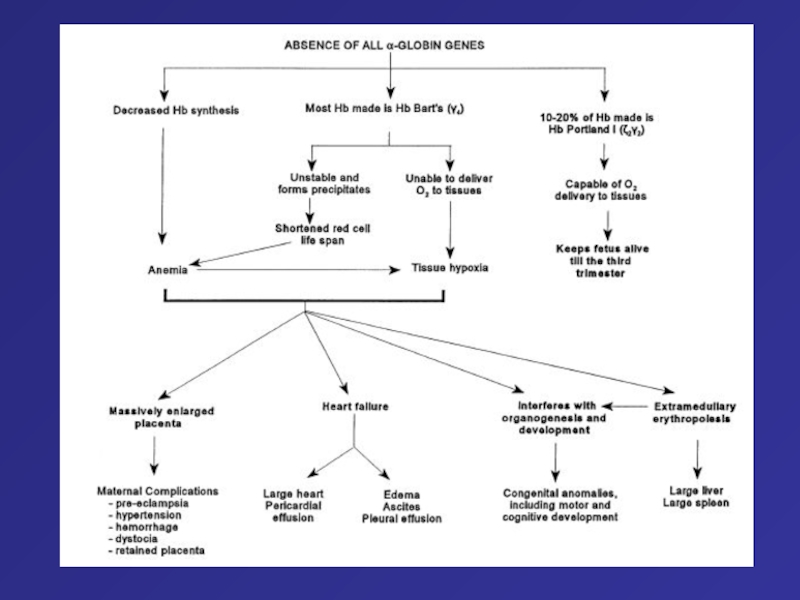
- 72. . Phenotypes of thalassaemias, sickle cell disease and various thalassaemia interactions
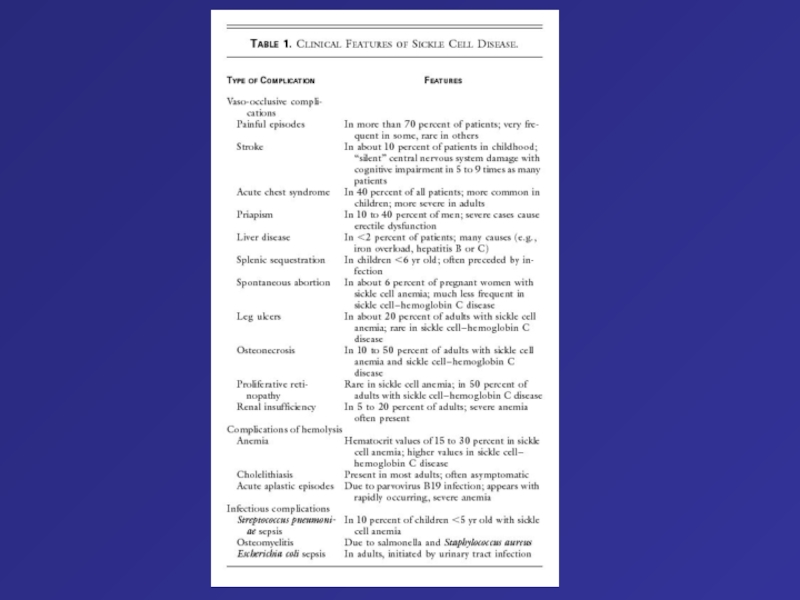
- 73. Clinical and Hematologic Features of the Principal Forms of Thalassemia a-Thalassemias
- 74. Слайд 74
- 75. Autopsy photographs of a newborn with hemoglobin
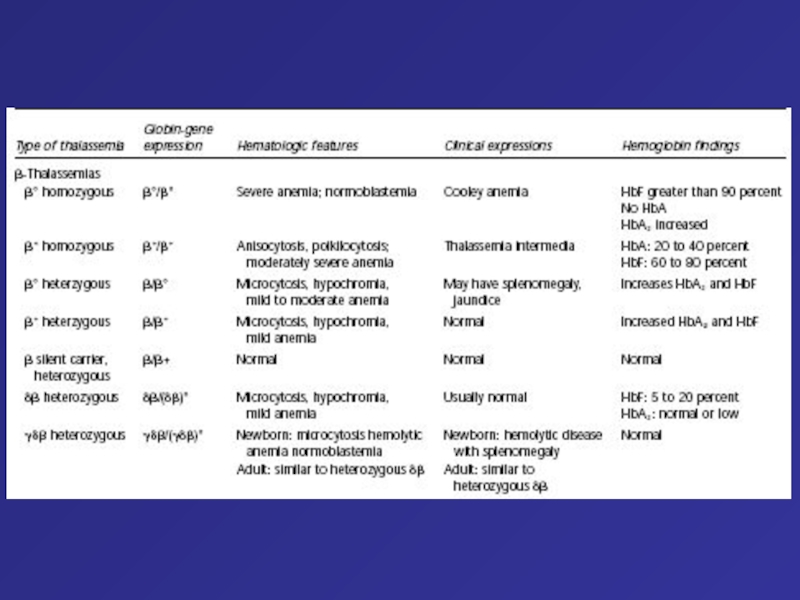
- 76. Type of thalassemia Globin-gene expression Hematologic features
- 77. Primary disease processes are indicated in orange,
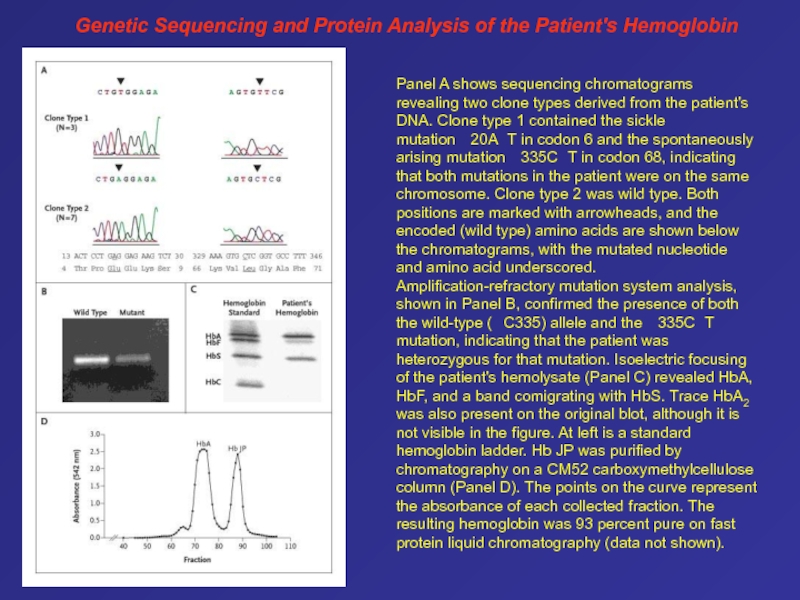
- 78. Genetic Sequencing and Protein Analysis of the
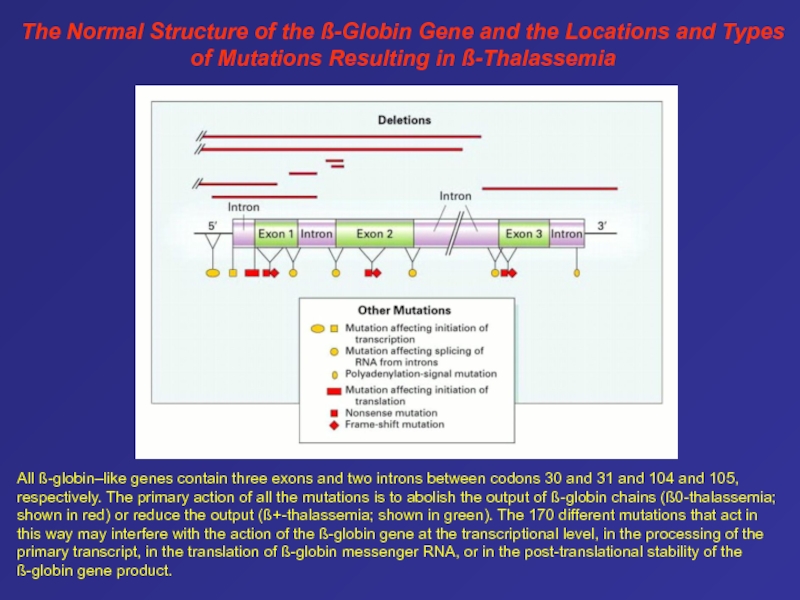
- 79. The Normal Structure of the ß-Globin Gene
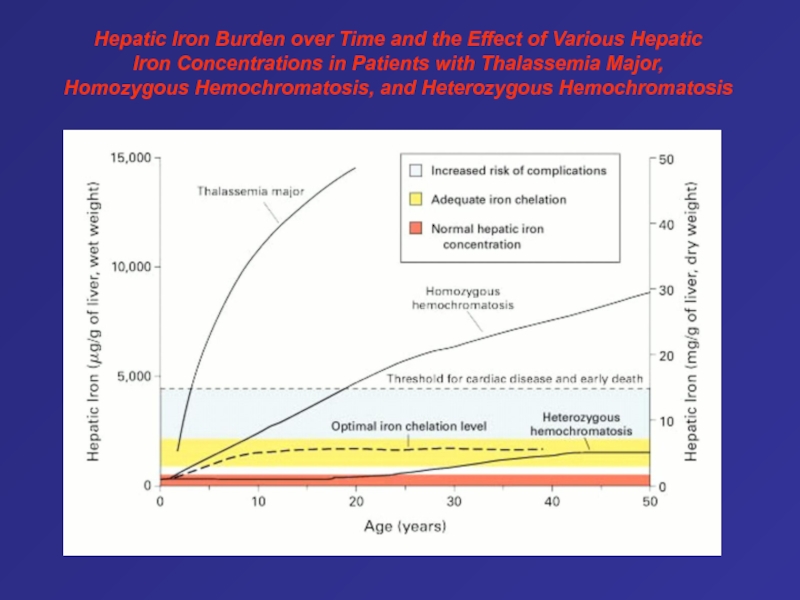
- 80. Hepatic Iron Burden over Time and the
- 81. Слайд 81
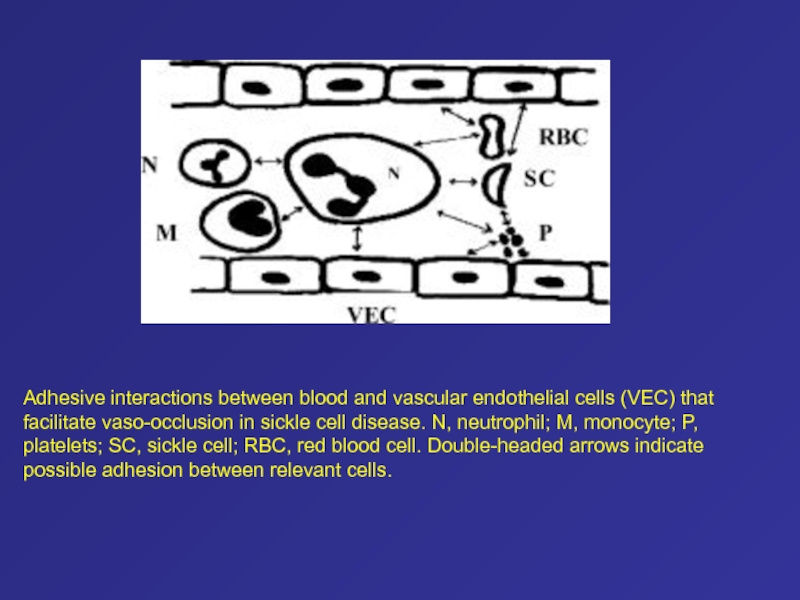
- 82. Adhesive interactions between blood and vascular endothelial
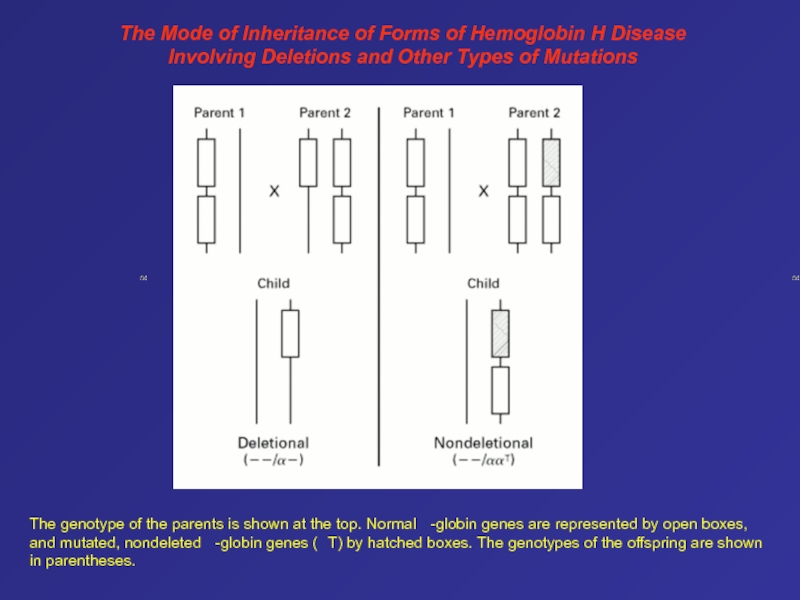
- 83. The Mode of Inheritance of Forms of
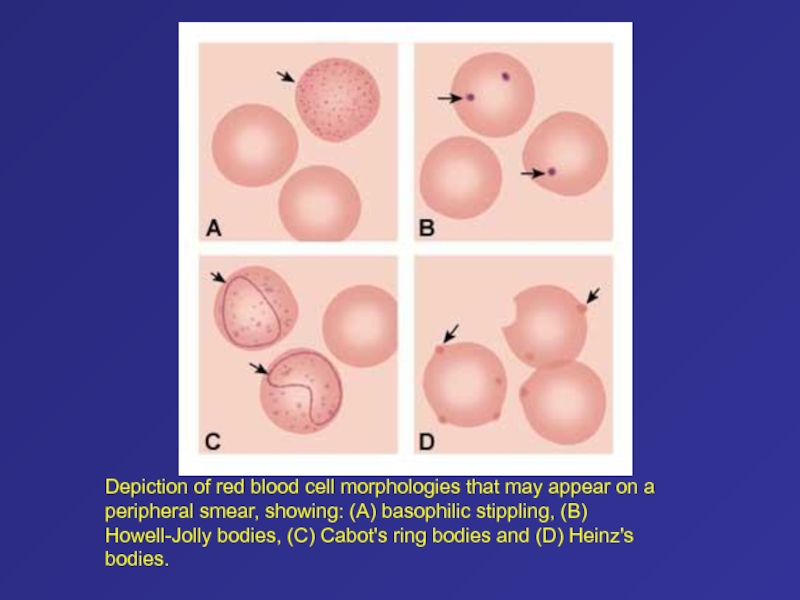
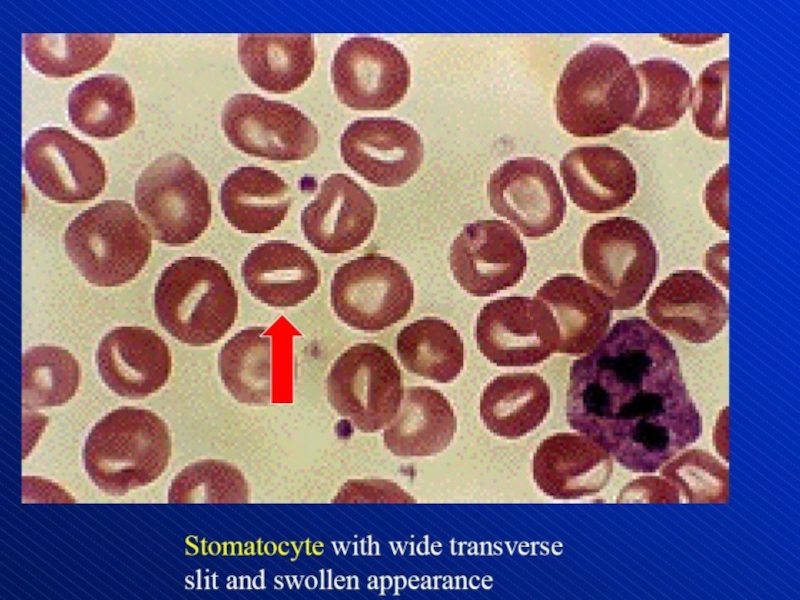
- 84. Depiction of red blood cell morphologies that
- 85. Слайд 85
- 86. Слайд 86
- 87. Слайд 87
- 88. Слайд 88
- 89. Слайд 89
- 90. Слайд 90
- 91. Слайд 91
- 92. Спасибо за внимание
- 93. Слайд 93
- 94. Слайд 94
- 95. Слайд 95
- 96. Слайд 96
- 97. Слайд 97
- 98. Слайд 98
- 99. Слайд 99
- 100. Слайд 100
- 101. Слайд 101
- 102. Слайд 102
- 103. Слайд 103
- 104. Слайд 104
- 105. Слайд 105
- 106. Слайд 106
- 107. Слайд 107
- 108. Скачать презентанцию
Слайды и текст этой презентации
Слайд 2Hemolytic anemia – increased destruction of erythrocytes
with adequate response of
bone marrow.
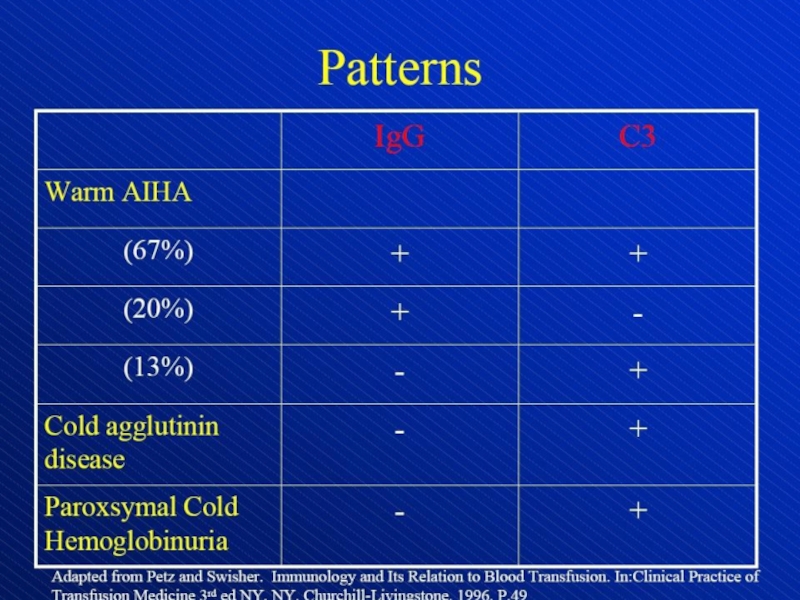
Слайд 13Direct antiglobulin test, demonstrating the presence of autoantibodies (shown here)
or complement on the surface of the red blood cell.
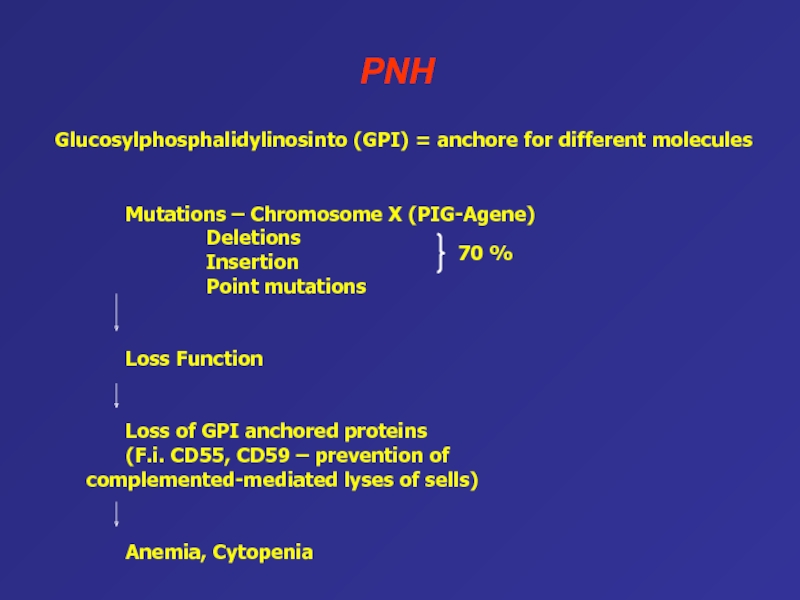
(RBCs = red blood cells)Слайд 52PNH
Mutations – Chromosome X (PIG-Agene)
Deletions
Insertion
Point mutations
Loss Function
Loss of GPI anchored
proteins
(F.i. CD55, CD59 – prevention of complemented-mediated lyses of sells)
Anemia,
CytopeniaGlucosylphosphalidylinosinto (GPI) = anchore for different molecules
70 %
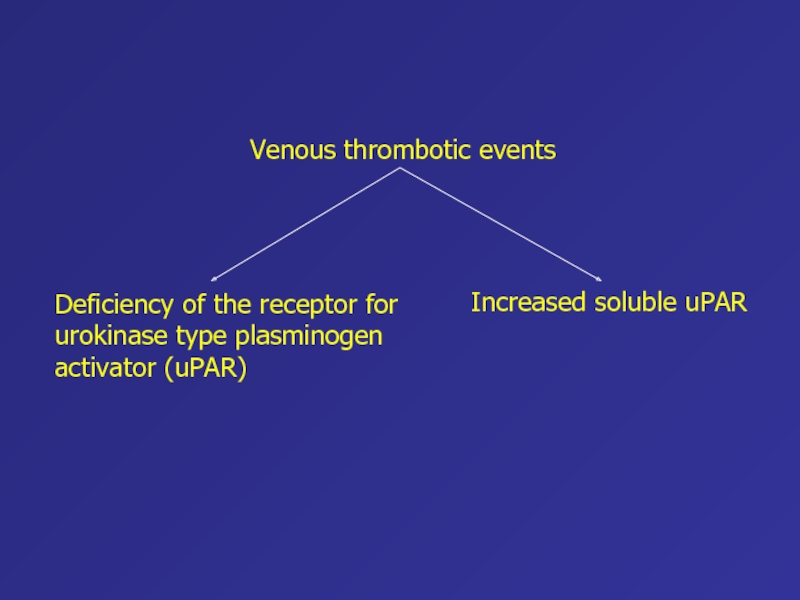
Слайд 54Increased soluble uPAR
Venous thrombotic events
Deficiency of the receptor for urokinase
type plasminogen activator (uPAR)
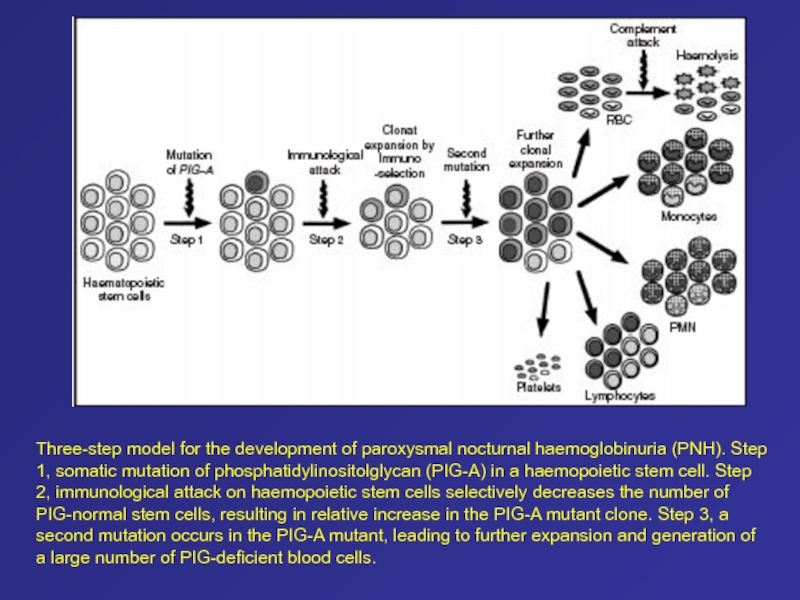
Слайд 57Three-step model for the development of paroxysmal nocturnal haemoglobinuria (PNH).
Step 1, somatic mutation of phosphatidylinositolglycan (PIG-A) in a haemopoietic
stem cell. Step 2, immunological attack on haemopoietic stem cells selectively decreases the number of PIG-normal stem cells, resulting in relative increase in the PIG-A mutant clone. Step 3, asecond mutation occurs in the PIG-A mutant, leading to further expansion and generation of a large number of PIG-deficient blood cells.
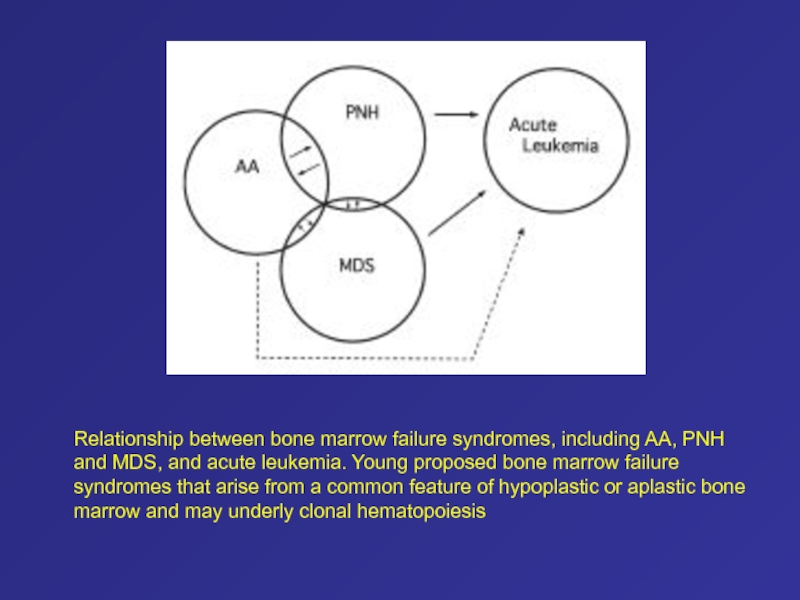
Слайд 58Relationship between bone marrow failure syndromes, including AA, PNH and
MDS, and acute leukemia. Young proposed bone marrow failure syndromes
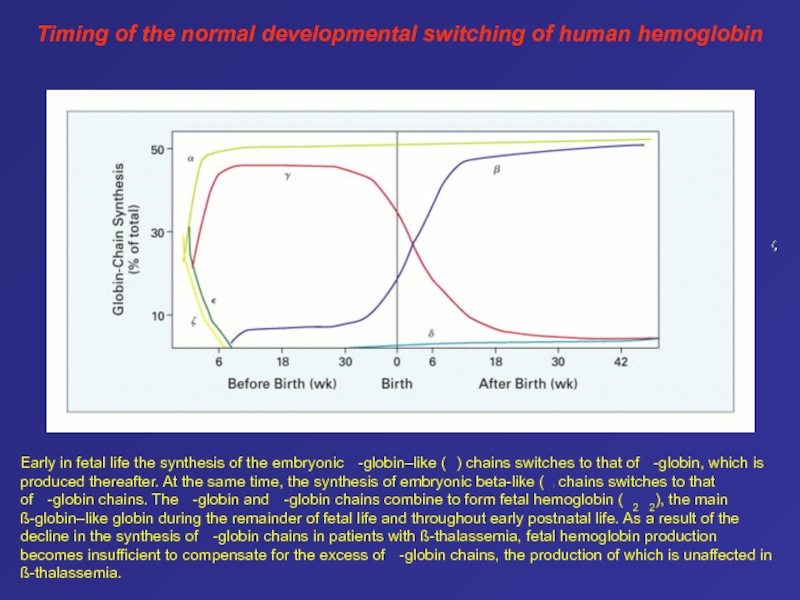
that arise from a common feature of hypoplastic or aplastic bone marrow and may underly clonal hematopoiesisСлайд 61Early in fetal life the synthesis of the embryonic
-globin–like ( ) chains switches to that of -globin,
which is produced thereafter. At the same time, the synthesis of embryonic beta-like ( ) chains switches to that of -globin chains. The -globin and -globin chains combine to form fetal hemoglobin ( 2 2), the main ß-globin–like globin during the remainder of fetal life and throughout early postnatal life. As a result of the decline in the synthesis of -globin chains in patients with ß-thalassemia, fetal hemoglobin production becomes insufficient to compensate for the excess of -globin chains, the production of which is unaffected in ß-thalassemia.Timing of the normal developmental switching of human hemoglobin
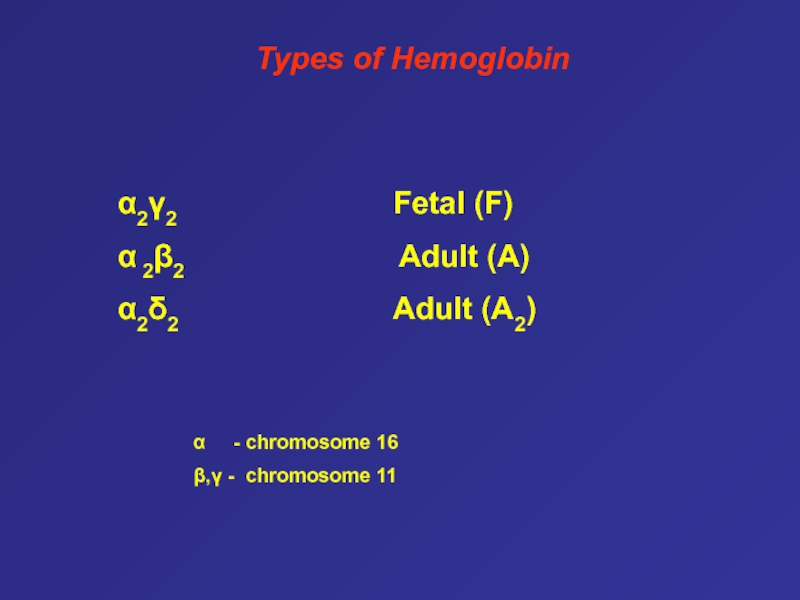
Слайд 62Types of Hemoglobin
α2γ2
Fetal (F)
α 2β2
Adult (A)α2δ2 Adult (A2)
α - chromosome 16
β,γ - chromosome 11
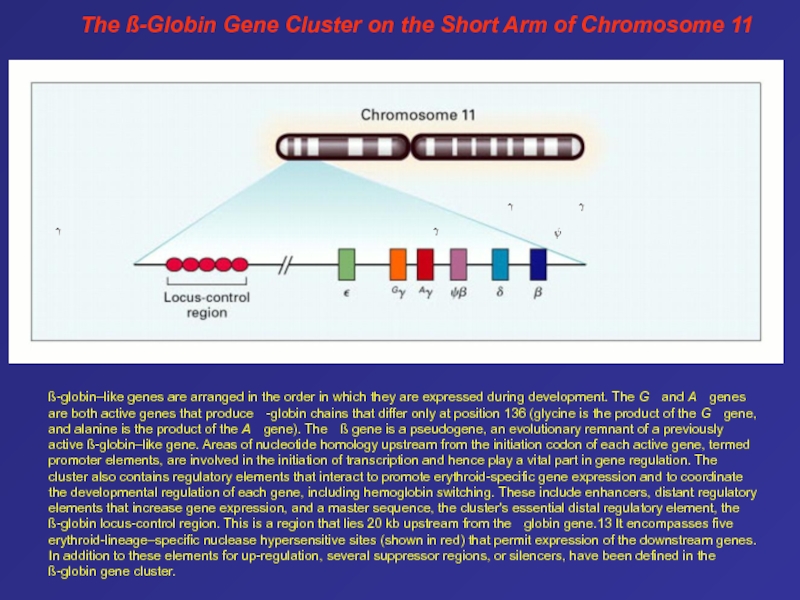
Слайд 63The ß-Globin Gene Cluster on the Short Arm of Chromosome
11
ß-globin–like genes are arranged in the order in which
they are expressed during development. The G and A genes are both active genes that produce -globin chains that differ only at position 136 (glycine is the product of the G gene, and alanine is the product of the A gene). The ß gene is a pseudogene, an evolutionary remnant of a previously active ß-globin–like gene. Areas of nucleotide homology upstream from the initiation codon of each active gene, termed promoter elements, are involved in the initiation of transcription and hence play a vital part in gene regulation. The cluster also contains regulatory elements that interact to promote erythroid-specific gene expression and to coordinate the developmental regulation of each gene, including hemoglobin switching. These include enhancers, distant regulatory elements that increase gene expression, and a master sequence, the cluster's essential distal regulatory element, the ß-globin locus-control region. This is a region that lies 20 kb upstream from the -globin gene.13 It encompasses five erythroid-lineage–specific nuclease hypersensitive sites (shown in red) that permit expression of the downstream genes. In addition to these elements for up-regulation, several suppressor regions, or silencers, have been defined in the ß-globin gene cluster. Слайд 64Pathophysiology of Sickle Cell Disease
In hemoglobin S, a substitution
of T for A in the sixth codon of the
ß-globin gene leads to the replacement of a glutamic acid residue by a valine residue. On deoxygenation, hemoglobin S polymers form, causing cell sickling and damage to the membrane. Some sickle cells adhere to endothelial cells, leading to vaso-occlusion.Слайд 65Desaturation of Normal and Variant Hemoglobins during Passage from Artery
to Vein
Hemoglobins lose oxygen during the journey from artery
to vein. Cells with HbS sickle only when desaturated. The likelihood that they will obstruct the vessel depends on the concentration of HbS and the extent of desaturation. Слайд 66This patient has homozygous sickle cell anemia, presenting with sickle
cell ulcers.1 Leg ulceration is a common complication in patients
with homozygous sickle cell anemia. The ulcers appear either spontaneously or as a result of local trauma, and they are chronic. Possible contributing factors are obstruction of vessels by sickled cells, increased venous and capillary pressure, bacterial infection and reduced oxygen-carrying capacity of the blood.1These ulcers are common in males, and their incidence increases significantly with advancing age. They occur more often in patients with lower hemoglobin levels and lower fetal hemoglobin levels; patients with SS and SS a-thalassemia have the highest ulcer prevalence rate of all genotypes. These ulcers occur with equal frequency over both medial and lateral aspects of the lower extremities.1 Treatment modalities include local ulcer care, antibiotics, skin grafting and transfusion therapy.1
Venous ulcers usually develop above the medial malleoli, in the area drained by incompetent perforating veins. These ulcers are well-demarcated with irregular shaggy borders and surrounding dermatitis, pigmentation and, sometimes, cellulitis.
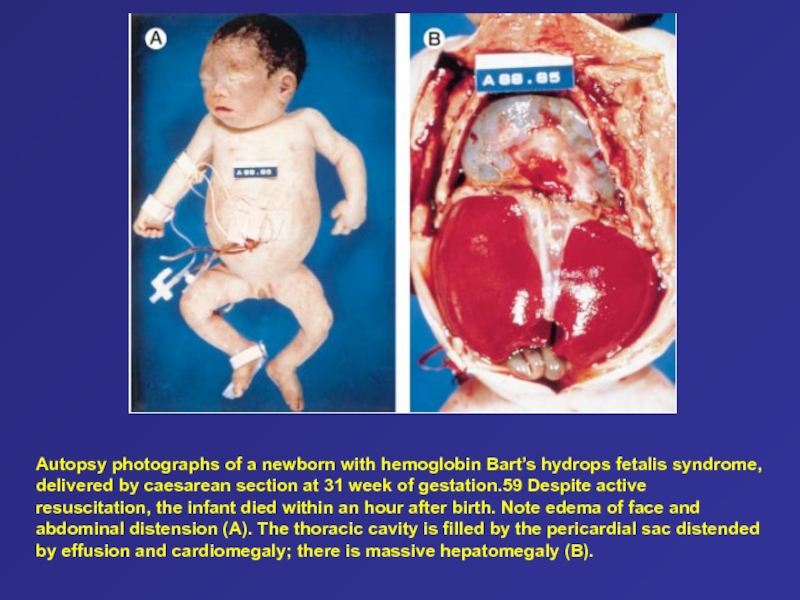
Слайд 75Autopsy photographs of a newborn with hemoglobin Bart’s hydrops fetalis
syndrome, delivered by caesarean section at 31 week of gestation.59
Despite active resuscitation, the infant died within an hour after birth. Note edema of face and abdominal distension (A). The thoracic cavity is filled by the pericardial sac distended by effusion and cardiomegaly; there is massive hepatomegaly (B).Слайд 76Type of thalassemia
Globin-gene expression
Hematologic features
Clinical expressions
Hemoglobin
findings
Clinical and Hematologic Features of the Principal Forms of
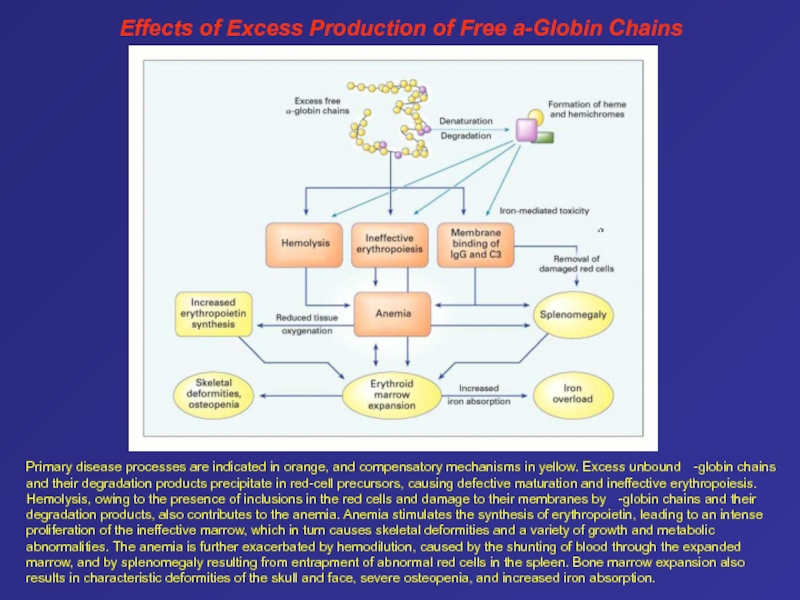
Thalassemia Слайд 77Primary disease processes are indicated in orange, and compensatory mechanisms
in yellow. Excess unbound -globin chains and their degradation
products precipitate in red-cell precursors, causing defective maturation and ineffective erythropoiesis. Hemolysis, owing to the presence of inclusions in the red cells and damage to their membranes by -globin chains and their degradation products, also contributes to the anemia. Anemia stimulates the synthesis of erythropoietin, leading to an intense proliferation of the ineffective marrow, which in turn causes skeletal deformities and a variety of growth and metabolic abnormalities. The anemia is further exacerbated by hemodilution, caused by the shunting of blood through the expanded marrow, and by splenomegaly resulting from entrapment of abnormal red cells in the spleen. Bone marrow expansion also results in characteristic deformities of the skull and face, severe osteopenia, and increased iron absorption.Effects of Excess Production of Free a-Globin Chains