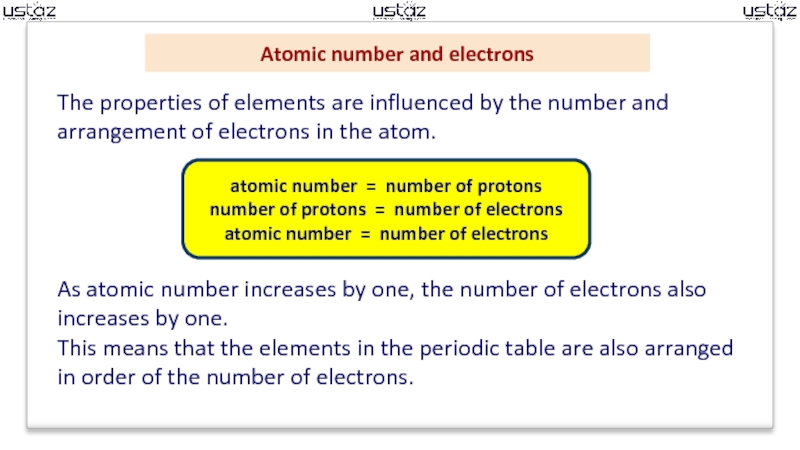
an atom. Sometimes called the proton number.
electron arrangement – A
shorthand way of writing the number of electrons in an atom’s electron shells.element – A substance made up of only one type of atom.
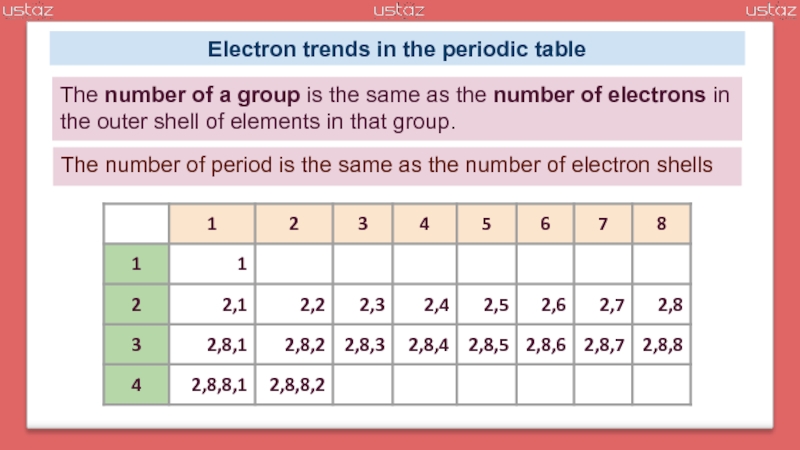
group – A column in the periodic table
period – A row in the periodic table containing elements with the same number of full electron shells.
periodic table – A table that lists all the elements in order of increasing atomic number
property – Any characteristic of an element.
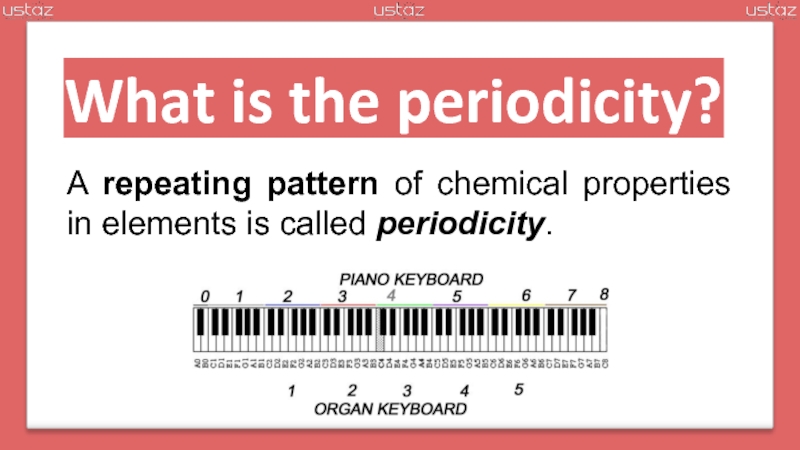
periodicity
pattern
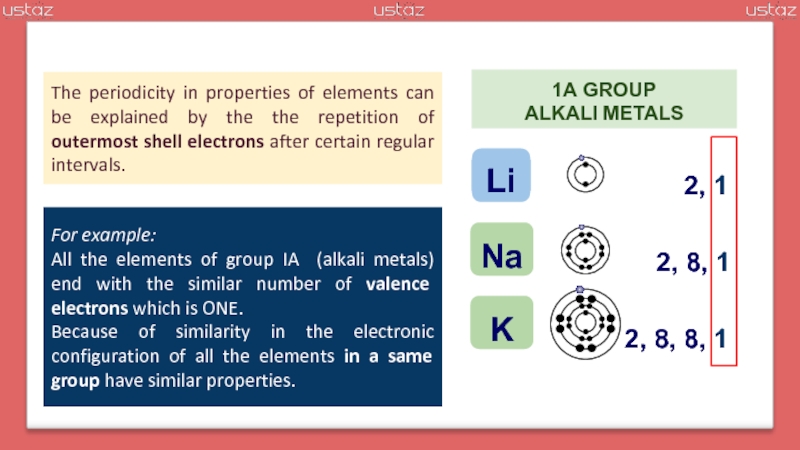
outermost
shell, subshell
valence electron
arrange, arrangement
consider
increase
distribute
belong
Make your own glossary