Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Vaccine Safety Epidemiology and Prevention of Vaccine-Preventable
Содержание
- 1. Vaccine Safety Epidemiology and Prevention of Vaccine-Preventable
- 2. Importance of Vaccine SafetyDecreases in disease risks
- 3. * Maximum cases reported in pre-vaccine
- 4. Importance of Vaccine SafetyOngoing safety monitoring needed for the development of sound policies and recommendations
- 5. Prelicensure Vaccine Safety StudiesLaboratoryAnimalsHumans
- 6. Prelicensure Human StudiesPhases I, II, III trialsCommon
- 7. Postlicensure SurveillanceIdentify rare reactionsMonitor increases in known
- 8. Postlicensure Vaccine Safety ActivitiesPhase IV Trials ~10,000 participantsbetter but still limitedLarge-Linked DatabasesClinical Immunization Safety Assessment Network
- 9. Vaccine Adverse Event Reporting System (VAERS) National
- 10. Vaccine Adverse Event Reporting System (VAERS) Detectsnew
- 11. Adverse Event ClassificationVaccine-inducedVaccine-potentiatedProgrammatic errorCoincidental
- 12. Vaccine Safety Datalink (VSD)Large-linked databaseLinks vaccination and
- 13. Clinical Immunization Safety Assessment (CISA) NetworkImprove understanding
- 14. Vaccine Injury Compensation Program (VICP)Established by
- 15. The Provider’s RoleImmunization providers can help to
- 16. The Provider’s RoleImmunization providers can help to
- 17. Contraindication A condition in a recipient that increases the chance of a serious adverse reaction
- 18. Precaution A condition in a recipient that
- 19. Invalid Contraindications to VaccinationMinor illnessMild/moderate local
- 20. Benefit and Risk CommunicationOpportunities for questions should
- 21. National Immunization Program Contact InformationTelephone 800.CDC.INFO Email nipinfo@cdc.gov Website www.cdc.gov/nip
- 22. Скачать презентанцию
Importance of Vaccine SafetyDecreases in disease risks and increased attention on vaccine risksPublic confidence in vaccine safety is criticalhigher standard of safety is expected of vaccinesvaccinees generally healthy (vs. ill
Слайды и текст этой презентации
Слайд 1Vaccine Safety
Epidemiology and Prevention of Vaccine-Preventable Diseases
National Immunization Program
Centers for
Disease Control and Prevention
Слайд 2Importance of Vaccine Safety
Decreases in disease risks and increased attention
on vaccine risks
Public confidence in vaccine safety is critical
higher standard
of safety is expected of vaccinesvaccinees generally healthy (vs. ill for drugs)
lower risk tolerance = need to search for rare reactions
vaccination universally recommended and mandated
Слайд 3* Maximum cases reported in pre-vaccine era
+ Estimated
because no national reporting existed in the prevaccine era
^ Adverse
events after vaccines against diseases shown on Table = 5,296** Invasive type b and unknown serotype
Comparison of Maximum and Current Reported Morbidity, Vaccine-Preventable Diseases and
Vaccine Adverse Events, United States
Слайд 4Importance of Vaccine Safety
Ongoing safety monitoring needed for the development
of sound policies and recommendations
Слайд 6Prelicensure Human Studies
Phases I, II, III trials
Common reactions are identified
Vaccines
are tested in thousands of persons before being licensed and
allowed on the marketСлайд 7Postlicensure Surveillance
Identify rare reactions
Monitor increases in known reactions
Identify risk factors
for reactions
Identify vaccine lots with unusual rates or types of
eventsIdentify signals
Слайд 8Postlicensure Vaccine Safety Activities
Phase IV Trials
~10,000 participants
better but still
limited
Large-Linked Databases
Clinical Immunization Safety Assessment Network
Слайд 9Vaccine Adverse Event Reporting System (VAERS)
National reporting system
Jointly administered
by CDC
and FDA
Passive (depends on healthcare providers and others
to report)Receives ~15,000 reports per year
Слайд 10Vaccine Adverse Event Reporting System (VAERS)
Detects
new or rare events
increases
in rates of known side effects
patient risk factors
Additional studies
required to confirm VAERS signalsNot all reports of adverse events are causally related to vaccine
Слайд 11Adverse Event Classification
Vaccine-induced
Vaccine-potentiated
Programmatic error
Coincidental
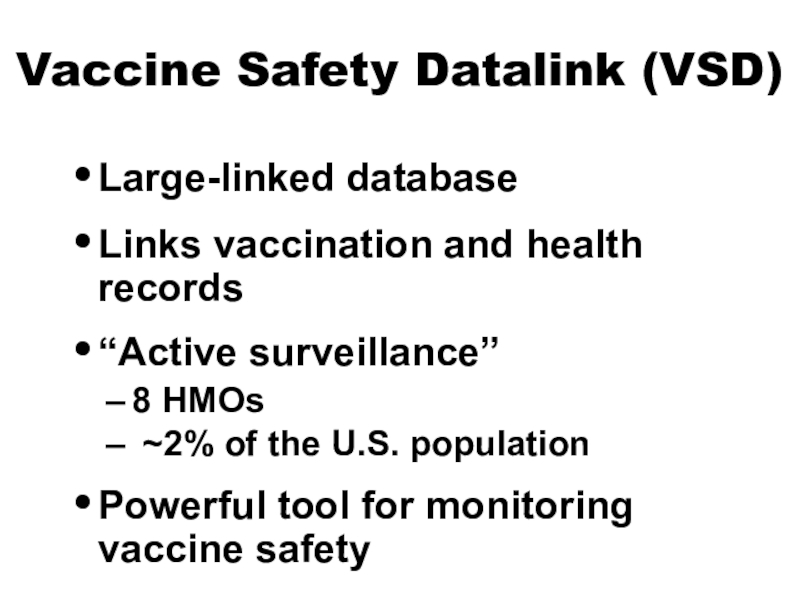
Слайд 12Vaccine Safety Datalink (VSD)
Large-linked database
Links vaccination and health records
“Active surveillance”
8
HMOs
~2% of the U.S. population
Powerful tool for monitoring vaccine
safetyСлайд 13Clinical Immunization Safety Assessment (CISA) Network
Improve understanding of vaccine safety
issues at individual level
Evaluate persons who experience adverse health events
Gain
better understanding of eventsDevelop protocols for healthcare providers
Слайд 14Vaccine Injury
Compensation Program (VICP)
Established by National Childhood Vaccine Injury
Act (1986)
“No fault” program
Covers all routinely
recommended childhood
vaccines
Vaccine Injury
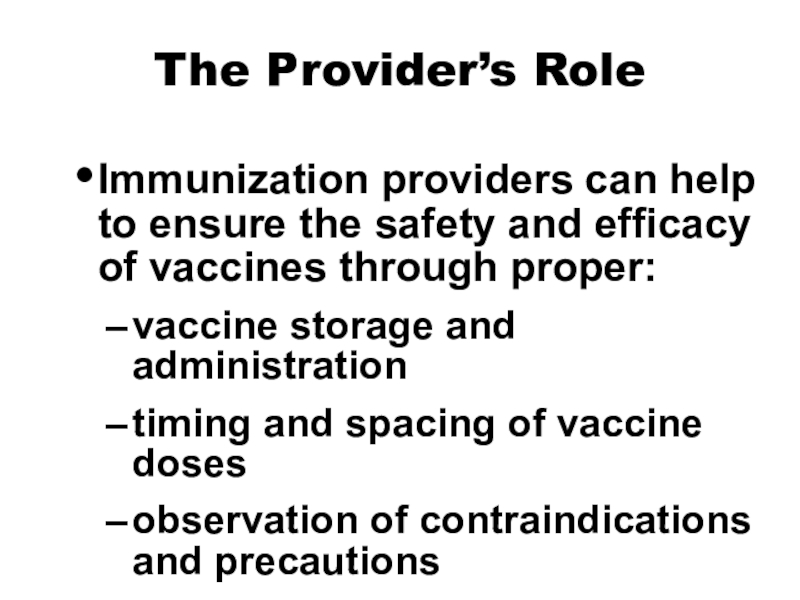
Table Слайд 15The Provider’s Role
Immunization providers can help to ensure the safety
and efficacy of vaccines through proper:
vaccine storage and administration
timing and
spacing of vaccine dosesobservation of contraindications and precautions
Слайд 16The Provider’s Role
Immunization providers can help to ensure the safety
and efficacy of vaccines through proper:
management of vaccine side effects
reporting
of suspected side effects to VAERSvaccine benefit and risk communication
Слайд 17Contraindication
A condition in a recipient that increases the
chance of a serious adverse reaction
Слайд 18Precaution
A condition in a recipient that might
Increase the
chance or severity of an adverse reaction, or
Compromise the ability
of the vaccine to produce immunityСлайд 19Invalid Contraindications
to Vaccination
Minor illness
Mild/moderate local reaction or fever following
a prior dose
Antimicrobial therapy
Disease exposure or convalescence
Pregnancy or immunosuppression in
the householdPremature birth
Breastfeeding
Allergies to products not in vaccine
Family history (unrelated to immunosuppression)
Слайд 20Benefit and Risk Communication
Opportunities for questions should
be provided before
each vaccination
Vaccine Information Statements (VISs)
must be provided before each dose
of vaccinepublic and private providers
available in multiple languages




















![Звуки [ p] и [ р’ ]
БУКВА Р
Подготовительная
группа](/img/tmb/6/585963/56560ced688c38d77a33d5a7dfeaec1c-800x.jpg)