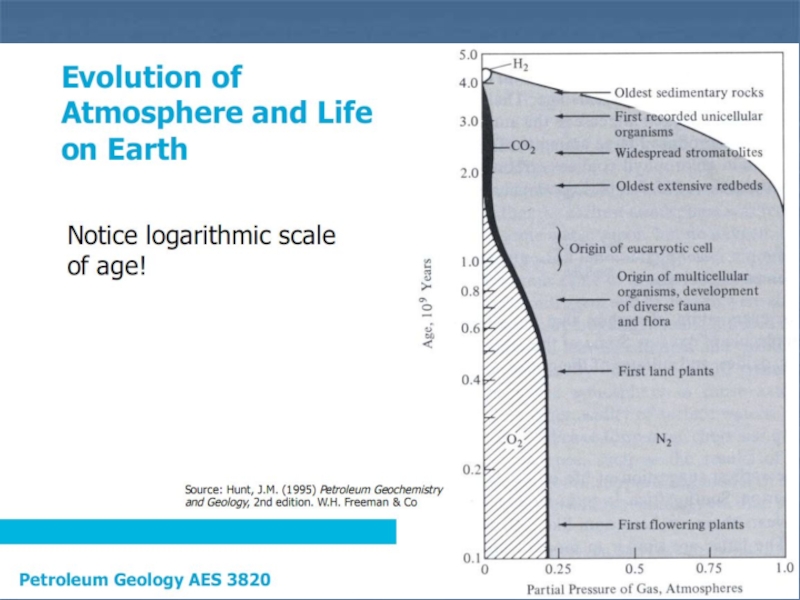
Слайд 12. The Carbon Cycle, Organic Matter and Maturation
Слайд 2Why is Carbon so Important
in the Life Cycle?
Carbon
has numerous ways of bonding with many other
elements, particularly
oxygen and hydrogen
It forms “organic” and “inorganic” compounds. Organic
compounds are considered unstable in the biosphere because
they are in the reduced state. Inorganic compounds, principally
calcite and dolomite, are stable because they are in the oxydized
state.
Carbon is contained in most substances that are vital for the
development of life (“biomolecules”): Proteins, lipids, sacharides,
etc.
Слайд 3Plankton is Main Source of Oil and Gas
Diatoms are
an important
group of phytoplankton. They
contain a silica skeleton
and
may reach 1 mm in diameter
(right). Other phytoplankton
organisms have a carbonate
skeleton.
Zooplankton includes planktonic
foraminifera, radiolaria, and
planktonic crustacea.
Слайд 4Planktonic Bloom in the Atlantic
Source: Scientific
American, June 2002
Слайд 7A Side Note on the Origin
of Petroleum
Many researchers
have proposed non-organic origins of
petroleum. Among these, the most
popular is the inorganic origin
theory. Russian geochemists proposed that telluric currents deep
in the Earth’s crust combine water, graphite, iron and sulfur as a
giant battery that were “cooked” into hydrocarbons. Porfirev
(1974) even postulated that all known oilfields were formed in this
way during the Neogene.
Another hypothesis was formulated by T. Gold. In his view, the
components of the early atmosphere are still stored in and slowly
degassed from the Earth’s mantle, mostly in the wake of
earthquakes.
Слайд 8A Side Note on the Origin of
Petroleum ctd.
Geochemical
evidence points strongly towards an organic
origin of petroleum, as
we shall see later in this course.
Theories of an inorganic origin mostly come from regions
where oil is found in igneous and metamorphic rocks, or where
no organic source rock is evident. One should not discard
these theories too lightly. After all, carbon is common in many
igneous rocks, and the early atmosphere was reducing and
probably rich in methane, in addition to water vapor, ammonia,
hydrogen and other hydrocarbons. It is possible that
photochemical reactions could modify it to form a heavy oil
slick on the Earth’s surface that formed a breeding ground for
more complex compounds including prebiotic forms.
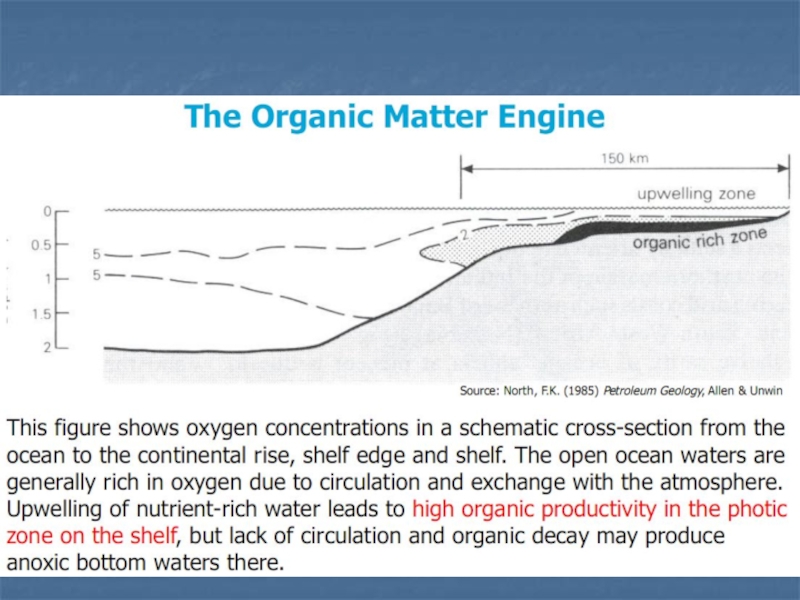
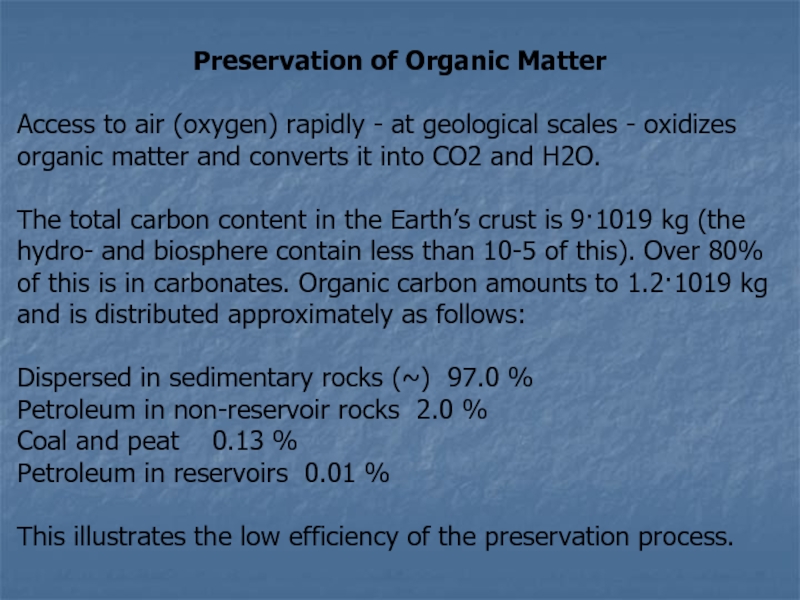
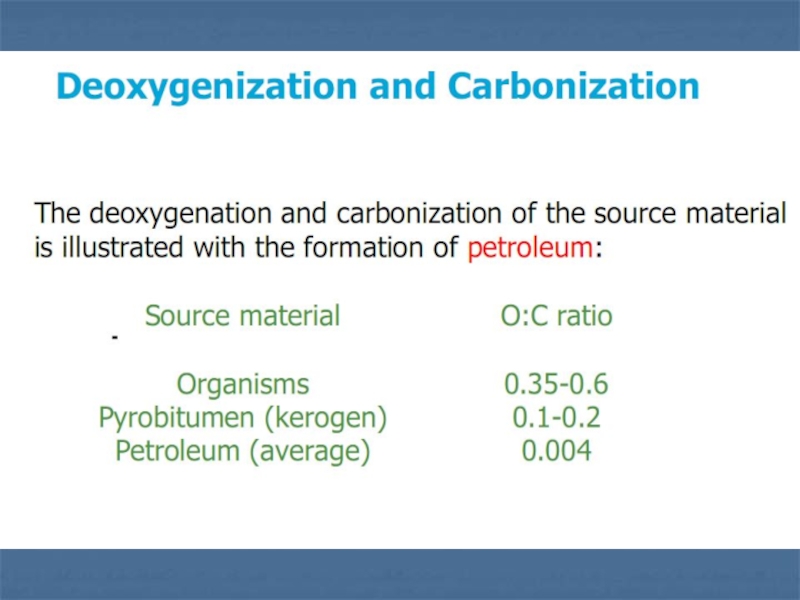
Слайд 12Preservation of Organic Matter
Access to air (oxygen) rapidly -
at geological scales - oxidizes organic matter and converts it
into CO2 and H2O.
The total carbon content in the Earth’s crust is 9·1019 kg (the hydro- and biosphere contain less than 10-5 of this). Over 80% of this is in carbonates. Organic carbon amounts to 1.2·1019 kg and is distributed approximately as follows:
Dispersed in sedimentary rocks (~) 97.0 %
Petroleum in non-reservoir rocks 2.0 %
Coal and peat 0.13 %
Petroleum in reservoirs 0.01 %
This illustrates the low efficiency of the preservation process.
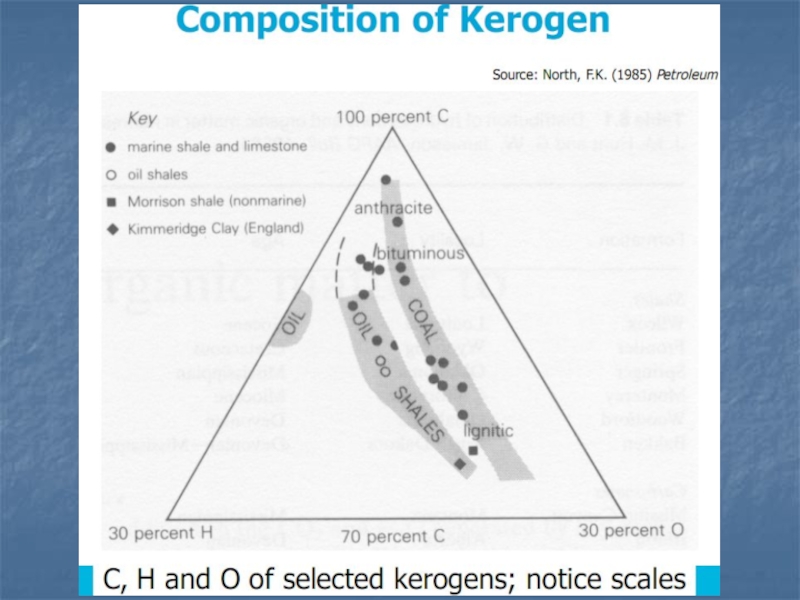
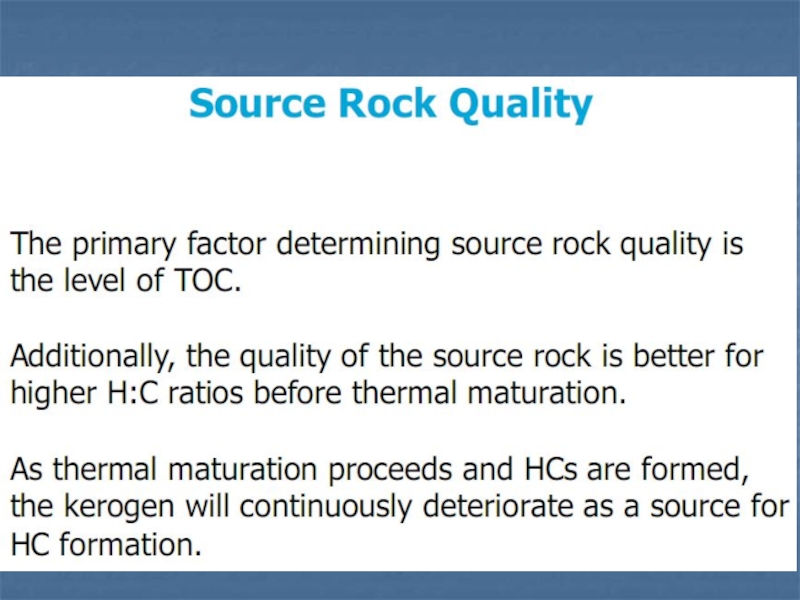
Слайд 13Total Organic Carbon (TOC)
If a rock contains significant amounts
of organic carbon, it is a
possible source rock for
petroleum or gas. The TOC content is
a measure of the source rock potential and is measured with
total pyrolysis.
The table below shows how TOC (in weight percent) relates to
the source rock quality.
TOC Quality
0.0-0.5 poor
0.5-1.0 fair
1.0-2.0 good
2.0-4.0 very good
>4.0 excellent
Слайд 14Source Rocks
Generally, finer-grained sediments contain more organic matter than
coarser-grained ones because of the restricted diffusion and thus the
lower amount of oxygen that can get in contact with OM. TOC can reach 20% or more. Coals and oil shales are rich in OM but are called source rocks.
A fine-grained carbonate rock of
Jurassic age containing abundant
organic matter.
Well Saddam-8, central Iraq.
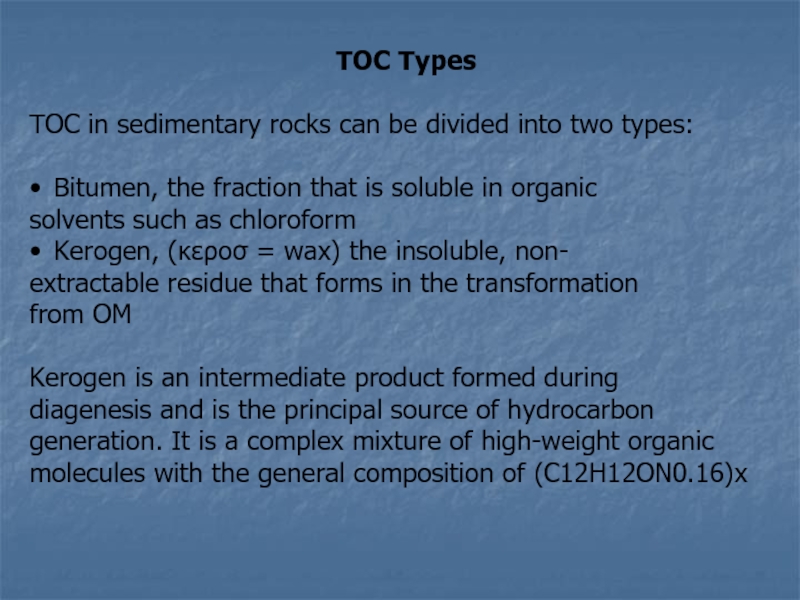
Слайд 16TOC Types
TOC in sedimentary rocks can be divided into
two types:
• Bitumen, the fraction that is soluble
in organic
solvents such as chloroform
• Kerogen, (κεροσ = wax) the insoluble, non-
extractable residue that forms in the transformation
from OM
Kerogen is an intermediate product formed during
diagenesis and is the principal source of hydrocarbon
generation. It is a complex mixture of high-weight organic
molecules with the general composition of (C12H12ON0.16)x
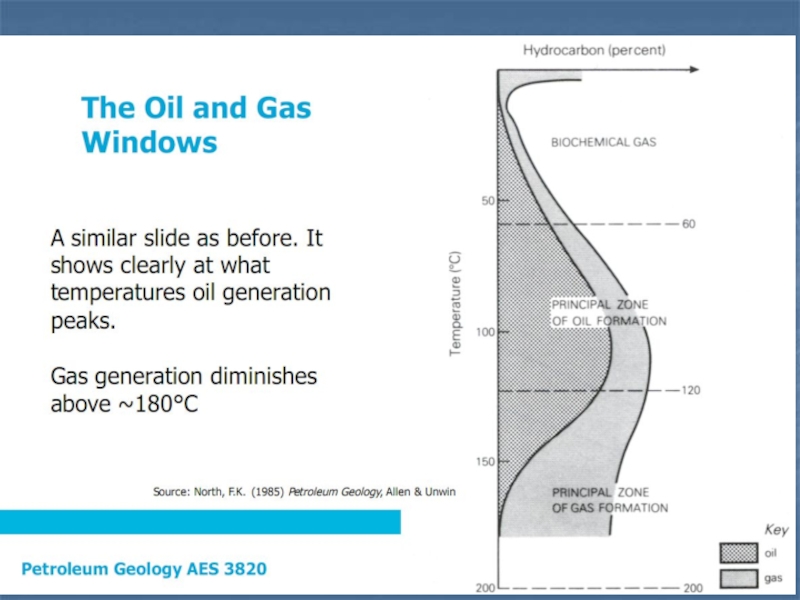
Слайд 20Temperature is the single most important factor in thermal maturation
Temperature
is the single most important factor in thermal maturation
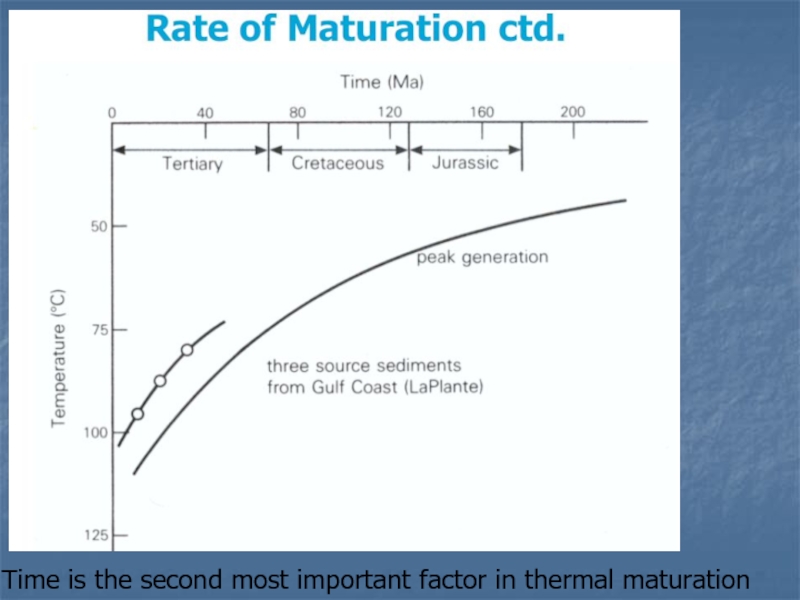
Слайд 21Time is the second most important factor in thermal maturation