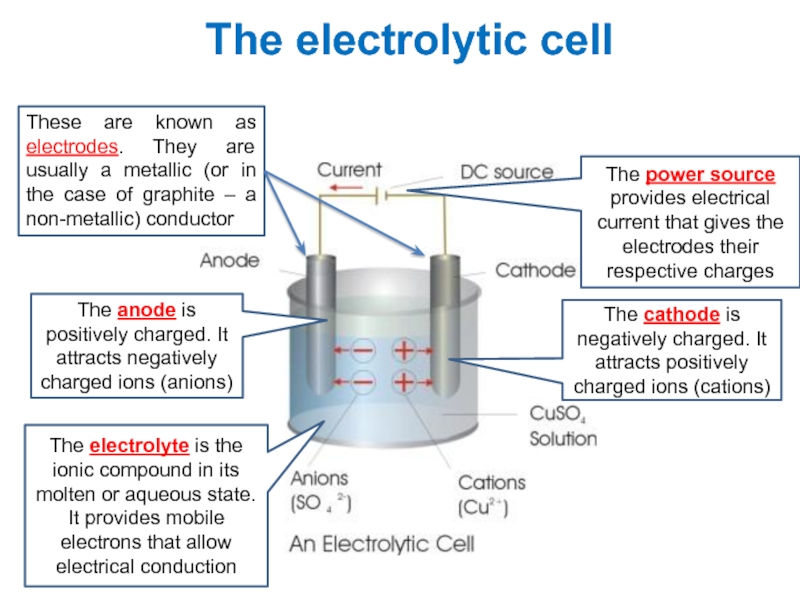
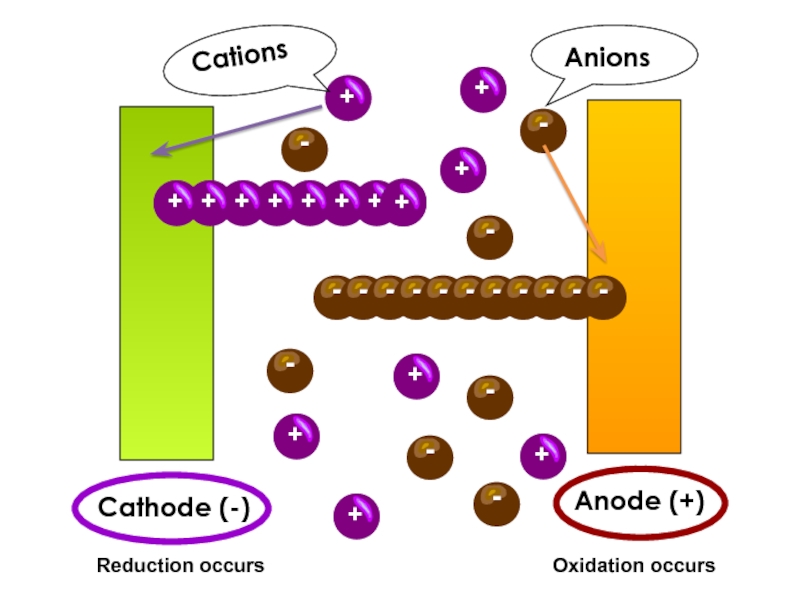
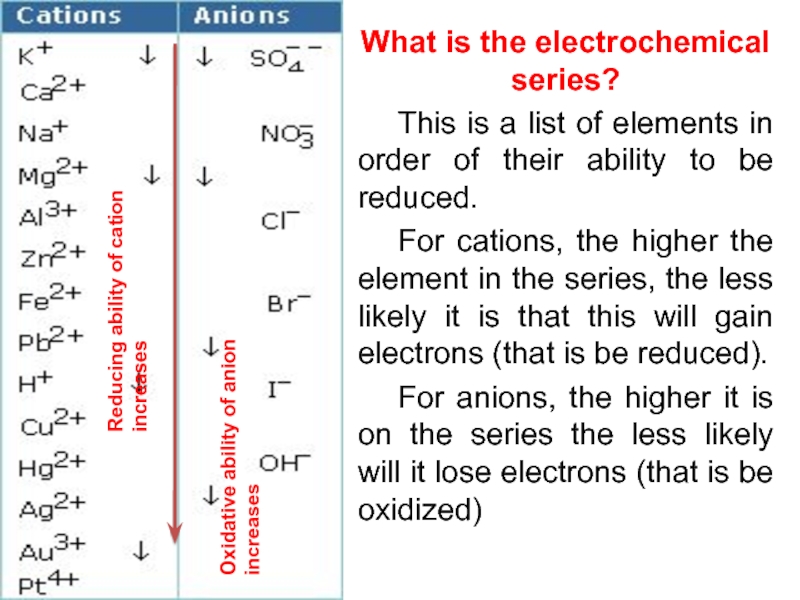
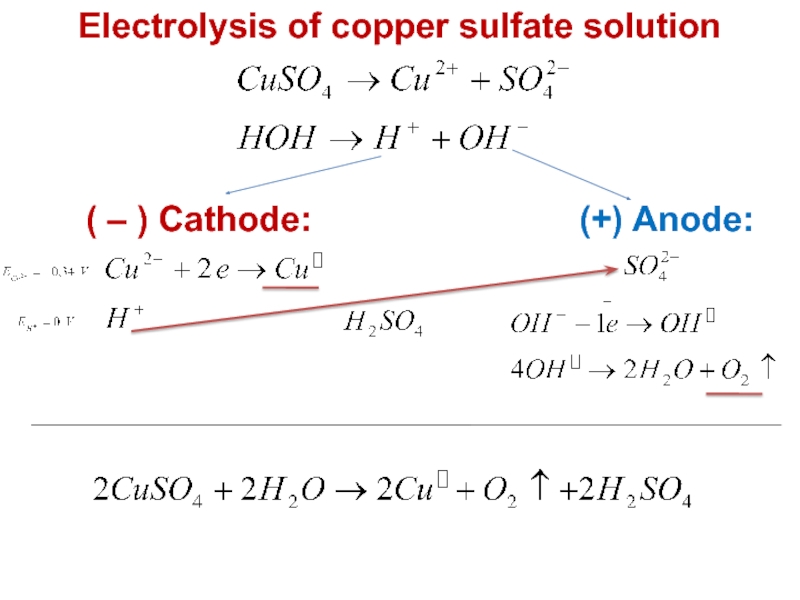
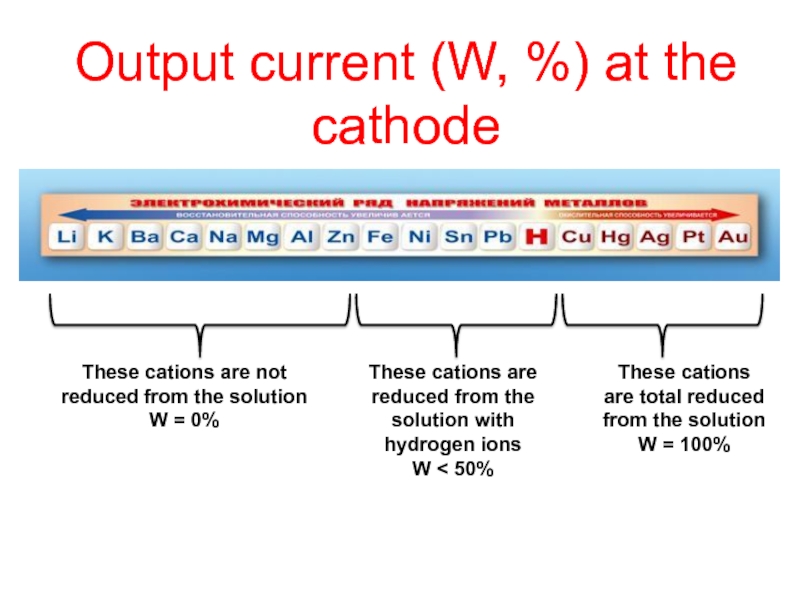
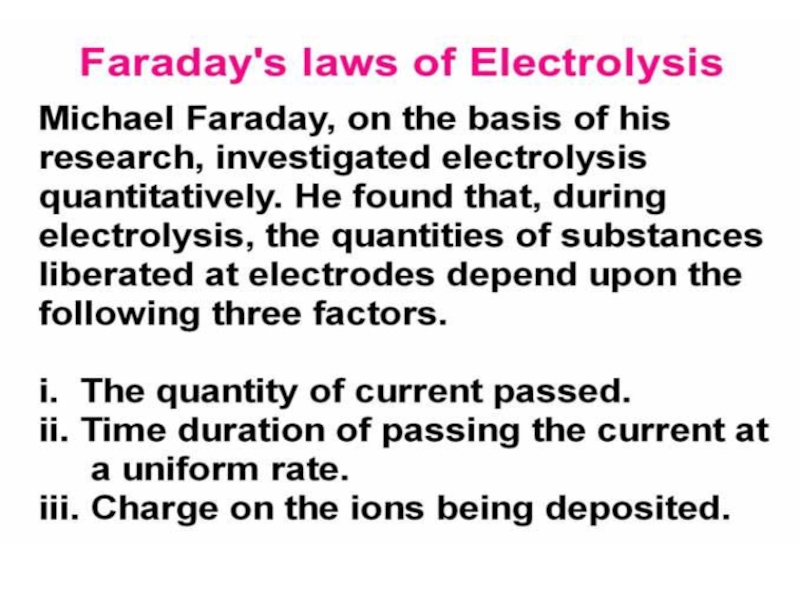
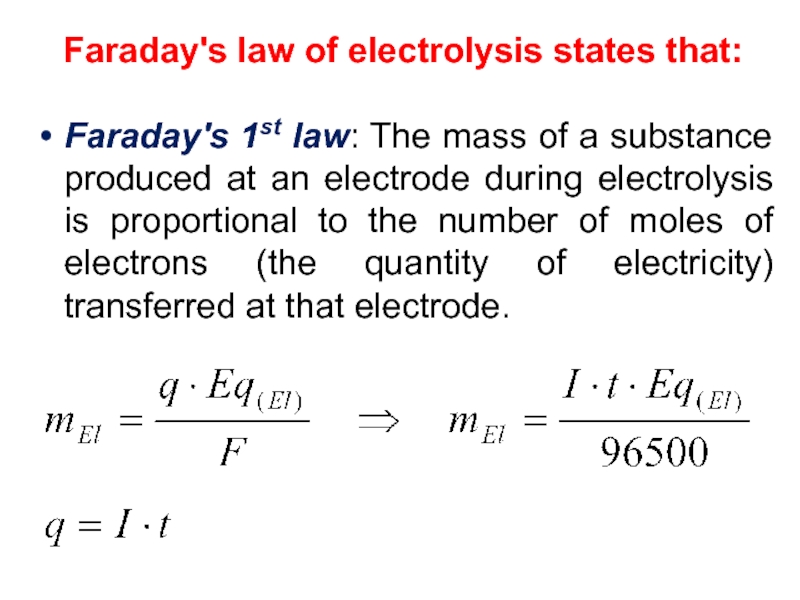
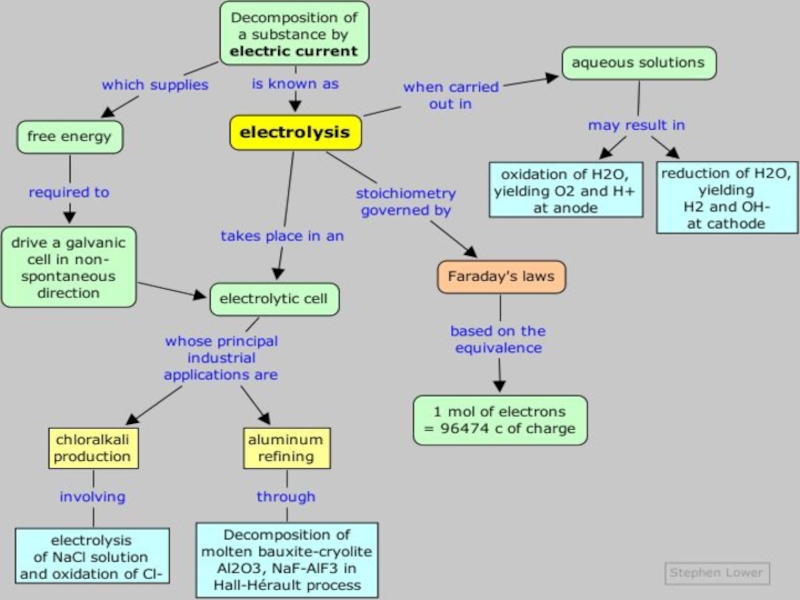
value of standard potential) is liberated first at the anode
if
anions are halogens i.e.
chloride Сl- , bromide Вr- and iodide I- the halogen is produced:
if – ions are not halogens eg sulphate SO42-, nitrate NO3-, carbonate CO32- and other, oxygen is produced, because OH- ion of water is electrolysed:
- CATHODE: the ion which is stronger oxidizing agent (high value of standard potential) is discharged first at the cathode
if cations (metals) are more reactive than hydrogen (before H atom in ecs):
K, Na, Ca, Mg, Zn, Fe ...... H2
then hydrogen is produced:
if cations (metals) are less reactive than hydrogen (after H atom in ecs): Cu, Ag, Au, Pt
then the metal is produced:
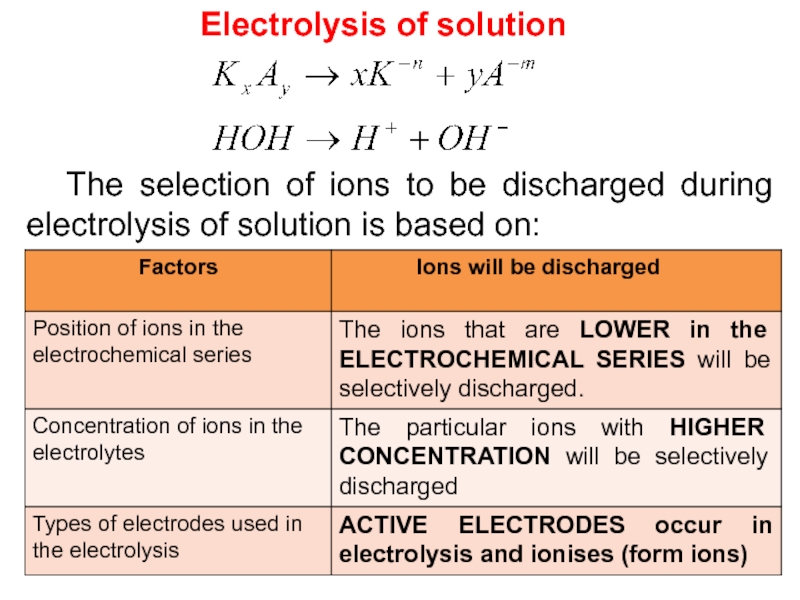
RULES FOR IONIC SOLUTIONS