Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Data Collection of Primary Central Nervous System (CNS) Tumors
Содержание
- 1. Data Collection of Primary Central Nervous System (CNS) Tumors
- 2. Portions of this presentation are based on
- 3. Part IRationaleHistoryDefinition of Reportable CasesCasefindingAnticipated Impact on Registries
- 4. Rationale for Non-malignant CNS Tumor Surveillance and
- 5. History 1992 -19961992 Central Brain Tumor Registry
- 6. History 1998BTWG forwarded four recommendations to the
- 7. BTWG Recommendations (1)The following standard definition is
- 8. BTWG Recommendations (2)Develop a standard site and
- 9. BTWG Recommendations (3)Develop training for reporting and
- 10. History 2000International Classification of Diseases for Oncology
- 11. History 2001-20022001 NCCCS Accepted Recommendations 1 and
- 12. Reportable Brain-Related Tumors (1)Public Law 107-260 requires
- 13. Reportable Brain-Related Tumors (2)Brain Cerebrum (C71.0)Frontal lobe (C71.1)Temporal lobe (C71.2)Parietal lobe (C71.3)Occipital lobe (C71.4).
- 14. Reportable Brain-Related Tumors (3)Brain (continued)Ventricle (C71.5)Cerebellum (C71.6)Brain stem (C71.7)Overlapping lesion of the brain (C71.8)Brain NOS (C71.9)
- 15. Reportable Brain-Related Tumors (4)Meninges Cerebral meninges (C70.0)Spinal meninges (C70.1)Meninges NOS (C70.9)Spinal cord (C72.0)Cauda equina (C72.1)
- 16. Reportable Brain-Related Tumors (5)Cranial nervesOlfactory nerve (C72.2)Optic nerve (C72.3)Acoustic nerve (C72.4)Cranial nerve NOS (C72.5)
- 17. Reportable Brain-Related Tumors (6)Other CNS (C72.8, C72.9)Pituitary
- 18. History 20032003 SEER-supported registries and COC-approved hospital
- 19. Impact of Collecting Data on Non-malignant CNS
- 20. Impact of Collecting Data on Non-malignant CNS
- 21. Impact of Collecting Data on Non-malignant CNS
- 22. Impact of Collecting Data on Non-malignant CNS
- 23. Case-finding (1)Additional or expanded case-finding mechanisms:Pathology RadiologyTreatment
- 24. Case-finding (2)Disease indicesSurgery logsDiagnostic imagingRadiation oncologyNeurology clinicsMedical oncologyAutopsy reports.
- 25. Case-finding SourcesFree-standing radiation therapy centersFree-standing Magnetic Resonance
- 26. ICD-9-CM Codes for Case-finding
- 27. Unusual and Ambiguous TerminologyIf the final pathologic
- 28. Part IICNS Anatomy and FunctionHistologies and Primary SitesGrading Systems and Coding Grade
- 29. CNS Functional AnatomySource: URL: www.solinas.com/solinas/brain.html accessed 7/18/03.
- 30. CNS AnatomyC71C71.6C71.7C72.0C71.0C75.3C75.1C71.7Source: URL: www.universalpeace.ca/principles.htm accessed 7/18/03.
- 31. Intracranial SitesC71.0C71.6C41.0C71.7C72.0Source: URL: mscenter.ucsf.edu/faq.htm accessed 7/18/03.Parietal lobeFrontal lobe
- 32. CerebrumC71.1C71.2C71.7C71.3C71.4C71.6C71.0Source: URL: www.sciencebob.com/lab/bodyzone/brainprint.html Accessed 7/18/03.
- 33. Cerebellum and Brain Stem C71.0C71.1C71.2C71.7C71.3C71.4C71.6URL: www.sciencebob.com/lab/bodyzone/brain.html 7/18/03
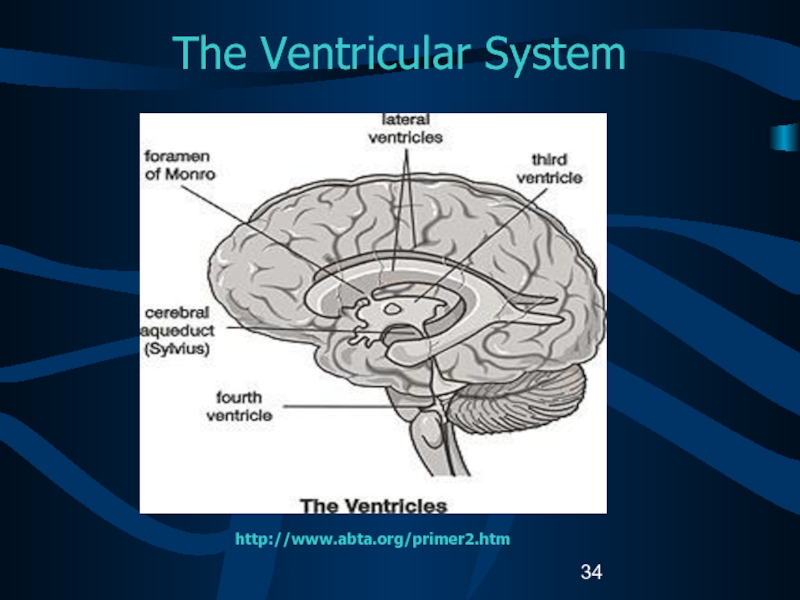
- 34. The Ventricular Systemhttp://www.abta.org/primer2.htm
- 35. Pineal and Pituitary GlandsC75.1C71.7C75.3C71.6C72.0Source: URL: training.seer.cancer.gov/module_anatomy/unit6_3_endo_gl… Accessed 7/18/03.
- 36. Cranial Nerves I=C72.2, II=C72.3, VIII=C72.4, Others=C72.5Source: URL: faculty.washington.edu/chudler/cranial.html Accessed 7/18/03.
- 37. Meninges C71.0C70.0C70.0Source: URL: www.cardioliving.com/consumer/Stroke/Hemorrhagic_Stroke.sht Accessed 7/18/03.
- 38. Tentorium C70.0C70.0Source: URL: neurosurgery.mgh.harvard.edu/abta/primer.htm Accessed 7/18/03.
- 39. Spinal Cord C72.0C70.1Source: URL: www.merck.com/pubs/mmanual/figures/182fig1.htm Accessed 7/18/03
- 40. Cellular ClassificationNeuroepithelial tumors AstrocytomasOligodendrogliomasEpendymomasPineal parenchymal tumorsOther CNS tumors Sellar tumorsHematopoetic tumorsGerm cell tumorsMeningiomasTumors of cranial nerves
- 41. Glial Tumors (1) Glial tissue: supportive tissue
- 42. Glial Tumors (2)Astrocytic tumors Noninfiltrating Juvenile pilocytic
- 43. Glial Tumors (3)Ependymal tumors Myxopapillary and well-differentiated
- 44. Glial Tumors (4)Mixed tumors Mixed astrocytoma-ependymomas Mixed astrocytoma-oligodendrogliomasMixed astrocytoma-ependymoma-oligodendrogliomas Other gliomasGanglioneuromas (M9490)Optic nerve gliomas
- 45. Non-Glial Tumors (1) Pineal region tumorsParenchymal tumorsPineocytomas
- 46. Non-Glial Tumors (2)MeningiomasMeningioma: Benign (M953_)Malignant meningiomasAnaplastic meningiomaHemangiopericytoma
- 47. Other CNS Tumors (1) Craniopharyngiomas (M9350)Rathke pouch tumorsChordomas (M9370)Schwannomas (M9560)Acoustic schwannomas/neuromas
- 48. Other CNS Tumors (2)Embryonal tumorsRetinoblastomas (M9510)Primitive neuroectodermal tumors (PNETs)Meduloblastomas (M9470) Neuroblastomas (M9500)
- 49. Other CNS Tumors (3)Lymphomas (M9590)Arise fromIndigenous brain
- 50. Other CNS Tumors (4) Cysts and tumor-like
- 51. Childhood versus Adult TumorsCNS tumor histology and
- 52. Childhood Brain TumorsMeduloblastomas are the most common
- 53. Cellular Classification Childhood Brain Tumors (1)Supratentorial
- 54. Cellular Classification Childhood Brain Tumors (2)The
- 55. Cellular Classification Childhood CNS TumorsCause of
- 56. ICD-O-3 Coding Issues (1)Some histologies may be
- 57. ICD-O-3 Coding Issues (2)Continue to assign histology
- 58. Grade for CNS TumorsSixth digit of ICD-O-3
- 59. WHO Grade (1)WHO grade coded in Collaborative
- 60. WHO Grade (2)Grade II Relatively slow growingSometimes
- 61. Kernohan GradeDefines progressive malignancy for astrocytomaGrade 1:
- 62. St. Anne-Mayo Grade (1)Used for astrocytomas. Uses
- 63. St. Anne-Mayo Grade (2)Grade 1: No criteriaGrade
- 64. Grade for CNS TumorsDo not record WHO
- 65. Part IIILateralityMultiple PrimariesMalignant TransformationSequence NumbersDate of Diagnosis
- 66. Determining Multiple Primaries: LateralityBrain is not
- 67. Coding Laterality (1)CNS sites to be coded
- 68. Coding Laterality (2)CNS sites to be coded
- 69. Determining Multiple Primaries: DefinitionsNon-malignant tumorTumor with ICD-O-3
- 70. Determining Multiple Primaries Malignant (1)NO CHANGES (at
- 71. Determining Multiple Primaries: Malignant (2)Histology Rule:
- 72. Determining Multiple Primaries Non-malignant (1)NEW RULESSite Rule:
- 73. Determining Multiple Primaries Non-malignant (2)Site (cont) EXCEPT
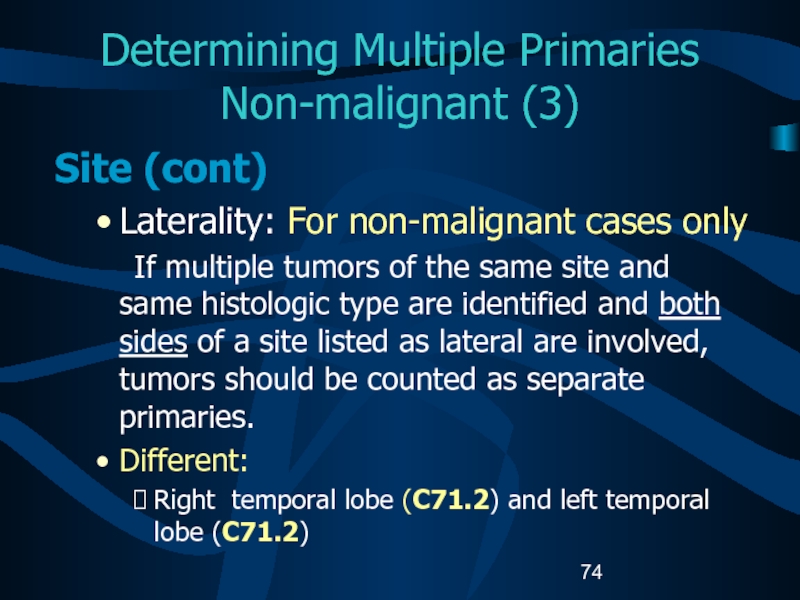
- 74. Determining Multiple Primaries Non-malignant (3)Site (cont) Laterality:
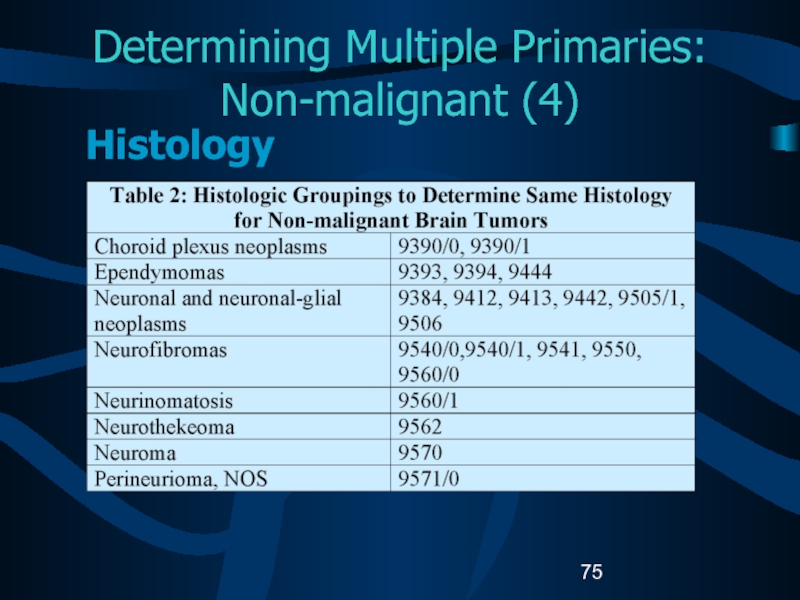
- 75. Determining Multiple Primaries: Non-malignant (4)Histology
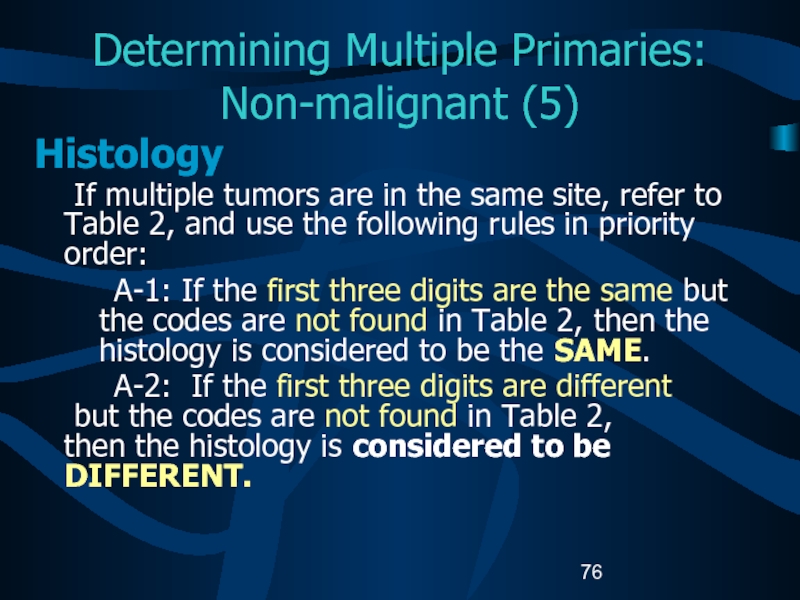
- 76. Determining Multiple Primaries: Non-malignant (5)Histology If multiple
- 77. Determining Multiple Primaries: Non-malignant (6)Histology (cont.) B.
- 78. Determining Multiple Primaries: Non-malignant (7)Histology (cont) C:
- 79. Determining Multiple Primaries: Non-malignant (8)Histology (cont) D:
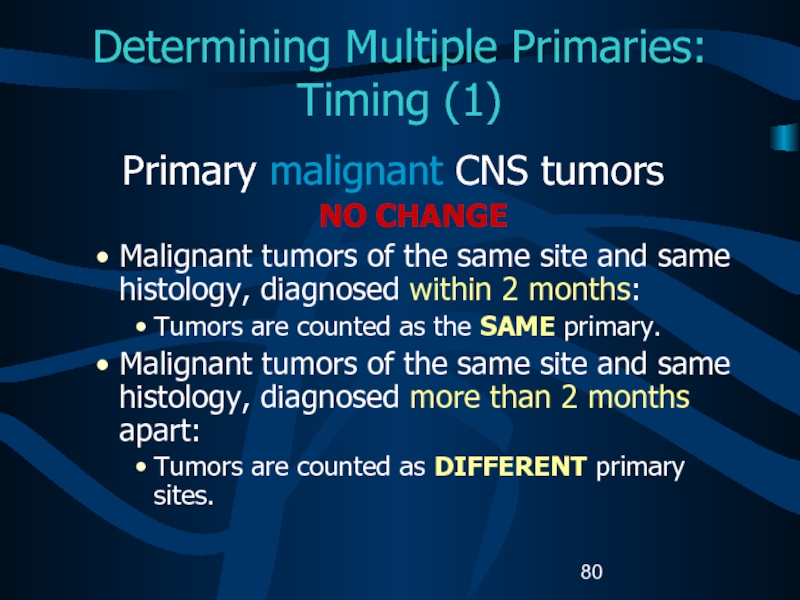
- 80. Determining Multiple Primaries: Timing (1)Primary malignant CNS
- 81. Determining Multiple Primaries: Timing (2)Primary non-malignant CNS
- 82. General Rules for Determining Multiple Primaries of
- 83. General Rules for Determining Multiple Primaries of
- 84. General Rules for Determining Multiple Primaries of
- 85. Histologic Transformation (1)Histologic transformation or progression to
- 86. Histologic Transformation (2)If a malignant CNS tumor
- 87. Histologic Transformation (3)Transformation of a non-malignant tumor
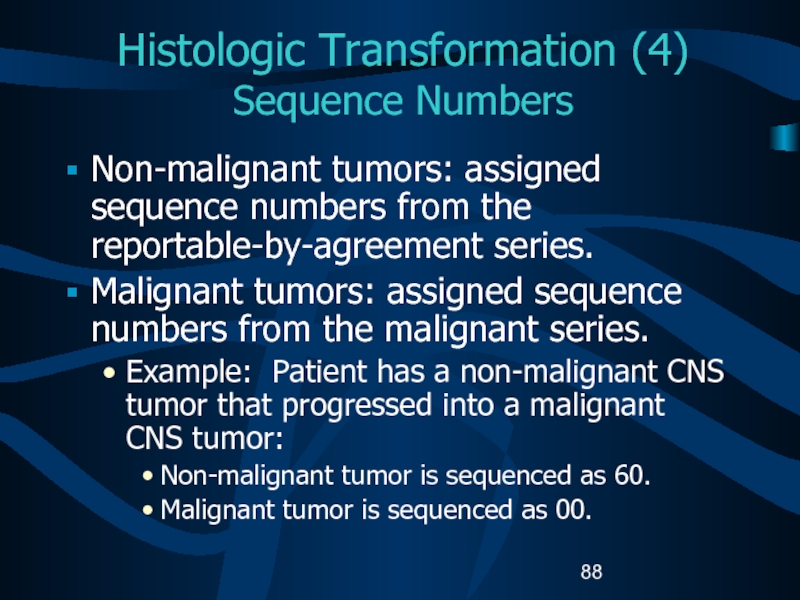
- 88. Histologic Transformation (4) Sequence NumbersNon-malignant tumors: assigned
- 89. Histologic Transformation (5) Date of DiagnosisNon-malignant tumors:
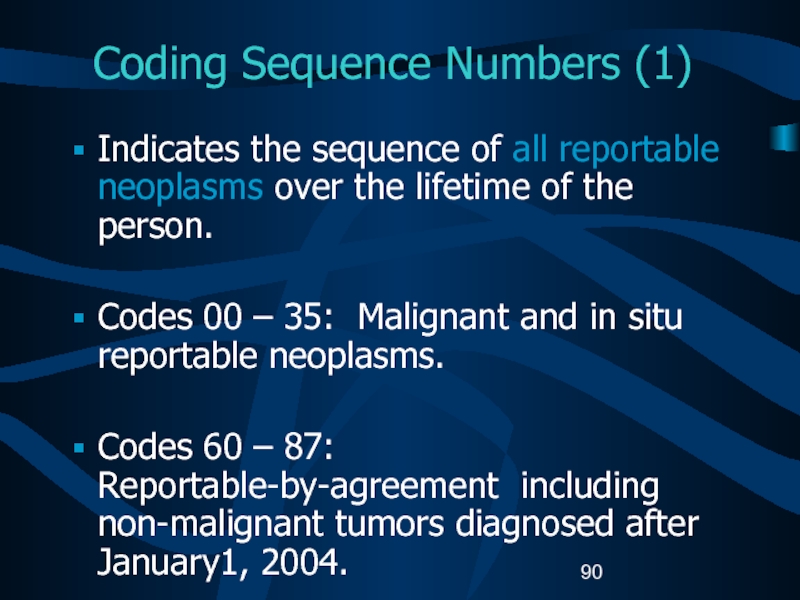
- 90. Coding Sequence Numbers (1)Indicates the sequence of
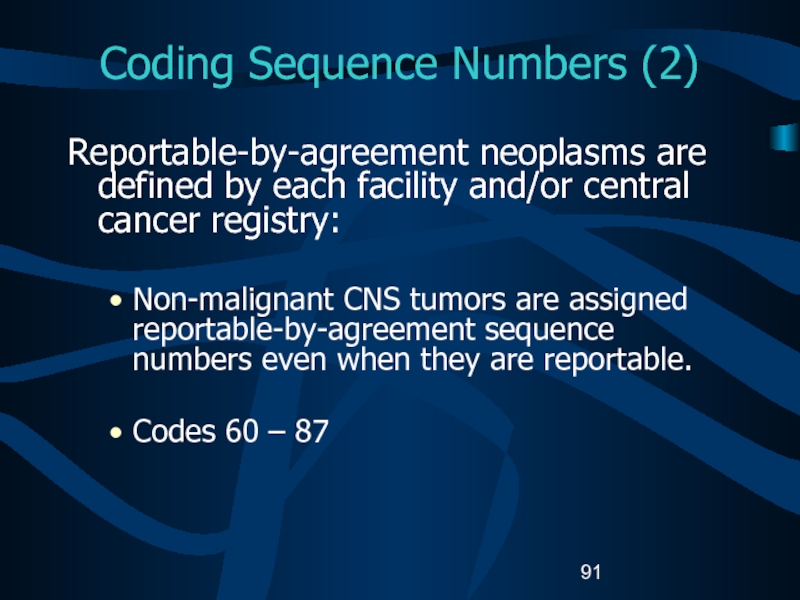
- 91. Coding Sequence Numbers (2)Reportable-by-agreement neoplasms are defined
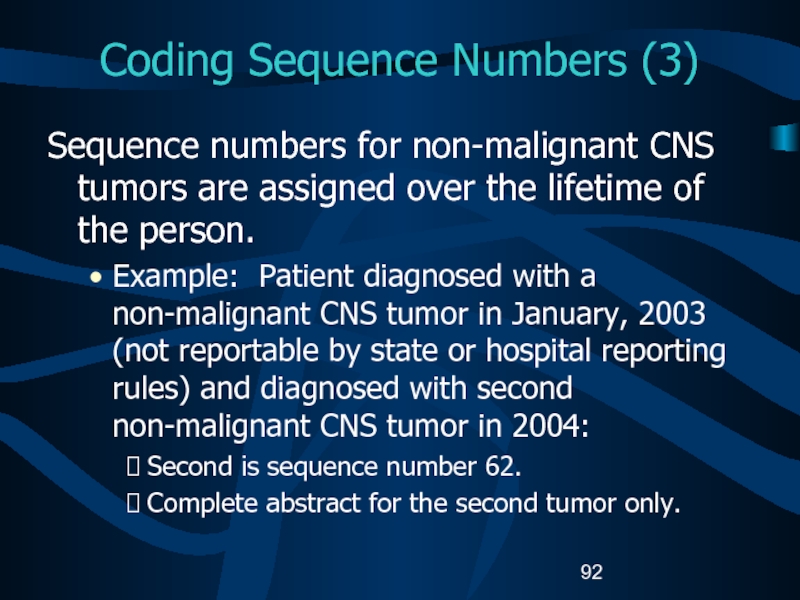
- 92. Coding Sequence Numbers (3)Sequence numbers for non-malignant
- 93. Assigning Diagnosis DateRules for assigning diagnosis date
- 94. Part IV StagingRisk Factors Genetic SyndromesDiagnostic ToolsTreatmentEdits Data Analysis
- 95. Collaborative Stage (CS)A computer algorithm uses the
- 96. Coding Collaborative Stage (1)Separate sets of extension
- 97. Coding Collaborative Stage (2)Site-specific codes for lymph
- 98. CS Extension: Brain and Meninges C70.0, C71.0
- 99. CS Extension: Brain and Meninges C70.0, C71.0
- 100. CS Extension: Brain and Meninges C70.0, C71.0
- 101. CS Extension: Brain and Meninges C70.0, C71.0
- 102. CS Extension: Other CNS C70.1-9, C72.0–C72.9
- 103. CS Extension: Other CNS C70.1-9, C72.0–C72.9 (2)05 Benign
- 104. CS Extension: Other CNS C70.1-9, C72.0–C72.9
- 105. CS Extension: Other Endocrine C75.1, C75.2,
- 106. CS Lymph Nodes Describes tumor involvement of regional
- 107. CS Metastasis at Diagnosis Brain and Meninges
- 108. CS Metastasis at Diagnosis Other CNS and
- 109. CS Site-specific Factor 1 (1) C70.0-C70.9,
- 110. CS Site-specific Factor 1 (2) C70.0-C70.9, C71.0-C71.9,
- 111. Possible Risk FactorsGenetic predispositions for the development
- 112. Possible Risk FactorsEpstein-Barr virus in the DNA
- 113. Genetic SyndromesGenetic syndromes associated with multiple CNS
- 114. Diagnostic Tools – Physical ExamNeurological examination Eye movementsVisionHearingReflexesBalance and coordinationSense of smell and touchAbstract thinkingMemory
- 115. Diagnostic Tools: RadiologyComputerized tomography (CT) scanMagnetic resonance
- 116. Diagnostic Tools: Laboratory testsAudiometryElectroencephalogram (EEG)Endocrine evaluationEvoked potentialsLumbar punctureMyelogramPerimetry
- 117. Diagnostic ToolsNeedle biopsyNeedle inserted through a burr
- 118. College of American Pathologist (CAP)
- 119. Brain and Spinal Cord CAP Protocols (1)MacroscopicSpecimen typeSpecimen sizeTumor siteTumor size
- 120. Brain and Spinal Cord CAP ProtocolsMicroscopicHistologic typeHistologic gradeMarginsAdditional studies*Additional pathologic findings*Comments**Not required for COC approval.
- 121. Treatment (1)Watchful waitingSurgery RadiationChemotherapyHormonal therapyImmunotherapyHematologic Transplant and Endocrine procedures
- 122. Treatment (2)Inoperable or inaccessible tumors may be
- 123. Surgical Procedure of Primary SiteBrain: Site-specific surgery
- 124. Surgical Procedure of Primary Site C70-0-C70.9, C71.0-C71.9,
- 125. Surgical Procedure of Primary Site C70-0-C70.9, C71.0-C71.9,
- 126. Surgical Procedure of Primary Site C75.1, C75.2,
- 127. Surgical Procedure of Primary Site C75.1, C75.2,
- 128. Surgical Procedure of Primary Site C75.1, C75.2,
- 129. Surgical Procedure of Primary Site C75.1, C75.2,
- 130. Surgical Margins of the Primary SiteCode final
- 131. Scope of Regional Lymph Node SurgeryIdentifies removal,
- 132. Radiation Therapy (1) Radiation codes indicate type
- 133. Radiation Therapy (2)Beam radiationCodes 20 – 29:
- 134. Radiation Therapy (3)Beam radiation Code 32: Conformal
- 135. Radiation Therapy (3)Tumors typically treated with stereotactic radiosurgery include: Acoustic neuromaChordomaPineal tumor AstrocytomaCraniopharyngiomaHemangioblastomaPituitary adenomal tumor
- 136. Radiation Therapy (4)Radioactive implantsCode 50: Brachytherapy, radiation
- 137. Radiation Therapy (5)Radioactive implants (continued)Code 52: Intracavitary
- 138. Chemotherapy (1)Record type of chemotherapy administered as
- 139. Chemotherapy (2)Blood-brain barrier Protects the brain from
- 140. Chemotherapy (3)Interstitial chemotherapyAdministered directly to involved tissues.Polymer
- 141. Hormone TherapyRecord systemic hormonal agents administered as
- 142. Immunotherapy (1)Record whether immunotherapeutic agents were administered
- 143. Immunotherapy (2)Gene therapy replaces or repairs the
- 144. Hematologic Transplant and Endocrine ProceduresIdentify systemic therapeutic
- 145. Technical Issues Edit ChecksNAACCR Edits Committee is
- 146. Technical Issues Data Analysis RecommendationsReport and analyze
- 147. ReferencesManuals, Articles, ReportsA Primer of Brain Tumors,
- 148. ReferencesManuals, Articles, Reports (continued)Fritz A, Percy C,
- 149. ReferencesWebsitesAmerican Brain Tumor Association www.abta.orgAmerican College of
- 150. ReferencesWebsites (continued)Brain and Neurosurgery Information Center www.brain-surgery.com/index.htmlBrain
- 151. ReferencesWebsites (continued)College of American Pathologists (CAP), Protocol
- 152. ReferencesWebsites (continued)International RadioSurgery Association www.isra.org/index.htmlNational Brain Tumor Radiosurgery Association www.braintumors.com/radiosurgery/radiosrugery.info#TWONCI Brain Tumor Home Page www.nci.nih.gov/cancer_information/cancer_type/brain_tumor/
- 153. ReferencesWebsites (continued)PDQ Cancer Information Summaries: Adult Treatment
- 154. Acknowledgments (1)Prepared byShannon Vann, CTRfor theNorth American
- 155. Acknowledgments (2)SponsorsCenters for Disease Control and PreventionNational
- 156. Acknowledgments (3)CDC National Program of Cancer Registries Planning CommitteeKimberly CantrellGayle G. ClutterFaye FloydMichael LanzilottaFrances Michaud
- 157. Acknowledgments (4)Materials Review CommitteeTrista Aarnes-Leong St. Vincent Medical
- 158. Скачать презентанцию
Portions of this presentation are based on non-malignant CNS tumor data collection rules adopted by the North American Association of Central Cancer Registries (NAACCR) Uniform Data Standards Committee - June 2003.
Слайды и текст этой презентации
Слайд 1Data Collection of Primary Central Nervous System (CNS) Tumors
DEPARTMENT OF
HEALTH AND HUMAN SERVICES
USAСлайд 2 Portions of this presentation are based on non-malignant CNS tumor
data collection rules adopted by the North American Association of
Central Cancer Registries (NAACCR) Uniform Data Standards Committee - June 2003.Слайд 3Part I
Rationale
History
Definition of Reportable Cases
Casefinding
Anticipated Impact on Registries
Слайд 4Rationale for Non-malignant CNS Tumor Surveillance and Registration
Non-malignant CNS tumors
cause disruption in normal function similar to that caused by
malignant CNS tumors.Location of a CNS tumor is as important as tumor behavior (benign or malignant) to morbidity and mortality.
Слайд 5History 1992 -1996
1992 Central Brain Tumor Registry of the United
States (CBTRUS) formed to report population-based data on primary benign,
borderline, and malignant CNS tumors.1996 National Coordinating Council on Cancer Surveillance (NCCCS) formed Brain Tumor Working Group (BTWG) to explore the feasibility of registering non-malignant CNS tumors
Слайд 6History 1998
BTWG forwarded four recommendations to the NCCCS
NCCCS
Accepted recommendations
1 and 2
Deferred recommendations 3 and 4
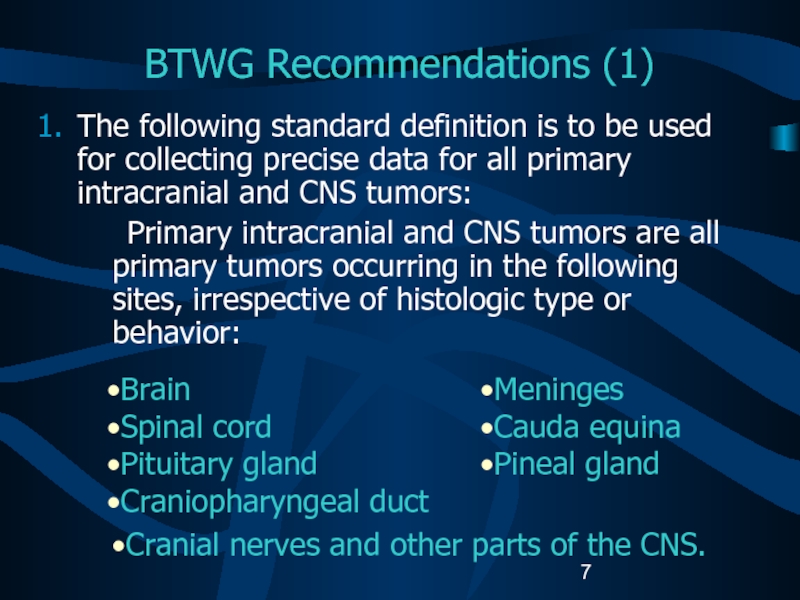
Слайд 7BTWG Recommendations (1)
The following standard definition is to be used
for collecting precise data for all primary intracranial and CNS
tumors:Primary intracranial and CNS tumors are all primary tumors occurring in the following sites, irrespective of histologic type or behavior:
Brain
Spinal cord
Pituitary gland
Craniopharyngeal duct
Meninges
Cauda equina
Pineal gland
Cranial nerves and other parts of the CNS.
Слайд 8BTWG Recommendations (2)
Develop a standard site and histology definition for
tabulating estimates of CNS tumors to allow comparability of information
across registries.All registries, both hospital- and population-based, should collect data on primary CNS tumors.
Слайд 9BTWG Recommendations (3)
Develop training for reporting and tabulating
primary intracranial and CNS tumors, and develop computerized edit- checking
procedures.Слайд 10History 2000
International Classification of Diseases for Oncology 3rd Edition (ICD-O-3)
and World Health Organization (WHO) 2000 Brain Tumor Classification are
compatible.November
Consensus conference on brain tumor definition convened. Group agrees to:
Site definition as in Recommendation 1.
Need to develop a standard site and histology definition based on the SEER site and histology validation list.
Слайд 11History 2001-2002
2001 NCCCS
Accepted Recommendations 1 and 2 as completed.
Reconvened
the BTWG to work on Recommendations 3 and 4.
2002 NAACCR
established subcommittee of Registry Operations Committee to:Identify changes needed in registry operations for inclusion of non-malignant CNS tumors.
October: Benign Brain Tumor Cancer Registries Amendment Act (Public Law 107-260) signed by President Bush.
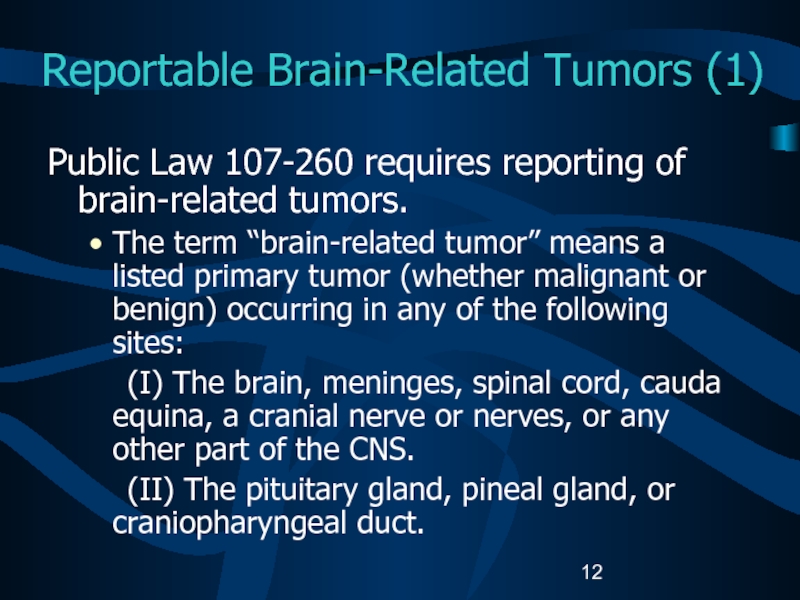
Слайд 12Reportable Brain-Related Tumors (1)
Public Law 107-260 requires reporting of brain-related
tumors.
The term “brain-related tumor” means a listed primary tumor (whether
malignant or benign) occurring in any of the following sites:(I) The brain, meninges, spinal cord, cauda equina, a cranial nerve or nerves, or any other part of the CNS.
(II) The pituitary gland, pineal gland, or craniopharyngeal duct.
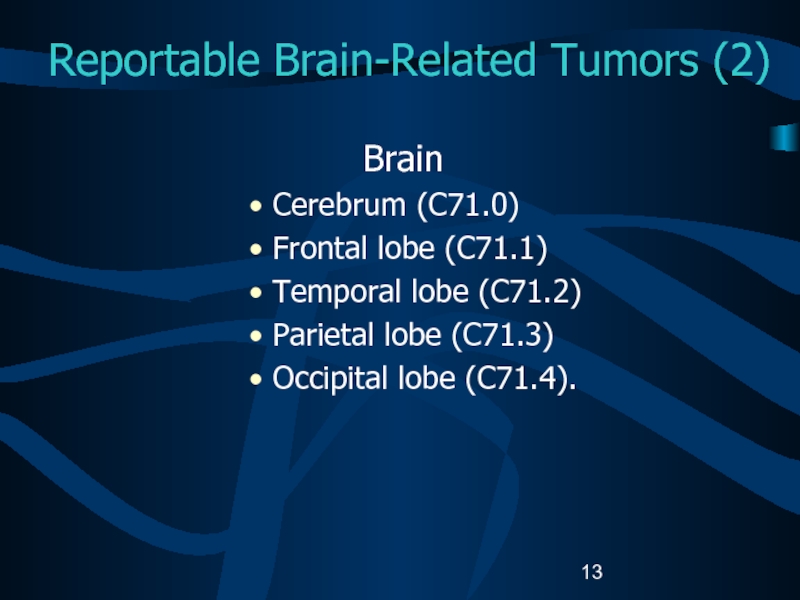
Слайд 13Reportable Brain-Related Tumors (2)
Brain
Cerebrum (C71.0)
Frontal lobe (C71.1)
Temporal lobe (C71.2)
Parietal
lobe (C71.3)
Occipital lobe (C71.4).
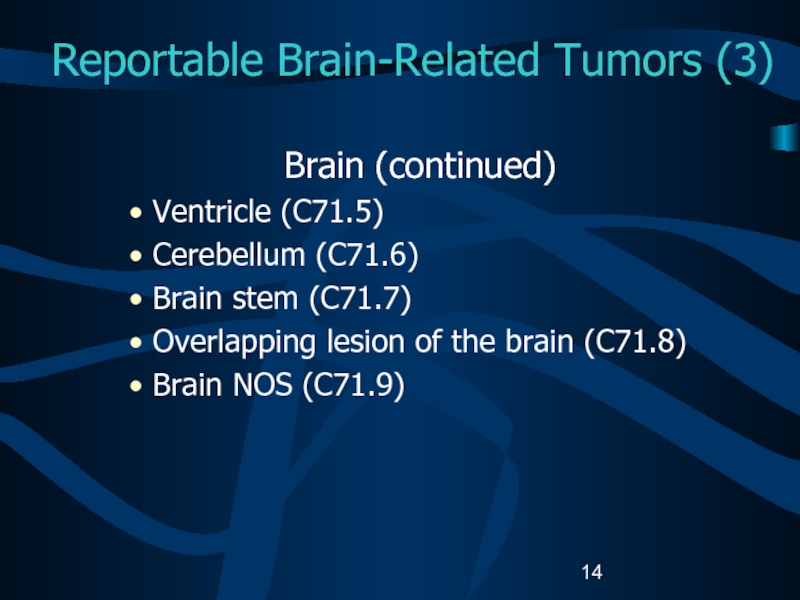
Слайд 14Reportable Brain-Related Tumors (3)
Brain (continued)
Ventricle (C71.5)
Cerebellum (C71.6)
Brain stem (C71.7)
Overlapping lesion
of the brain (C71.8)
Brain NOS (C71.9)
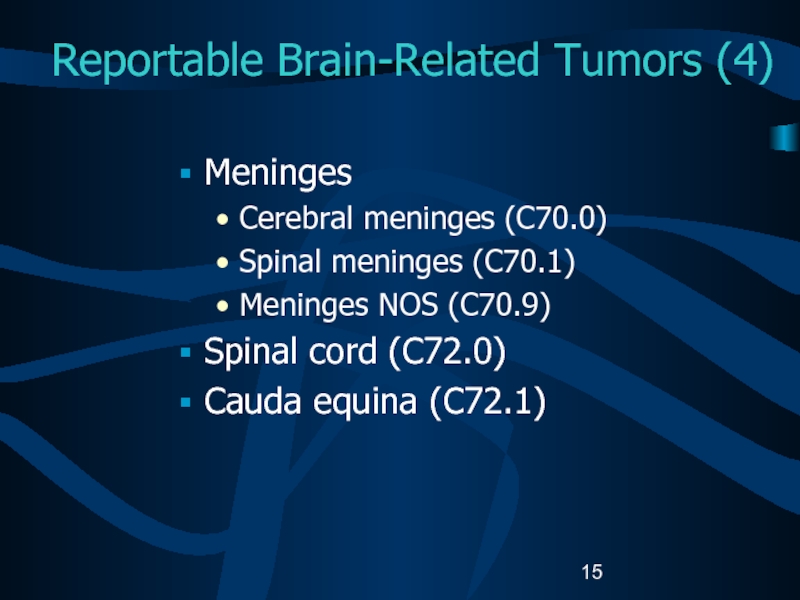
Слайд 15Reportable Brain-Related Tumors (4)
Meninges
Cerebral meninges (C70.0)
Spinal meninges (C70.1)
Meninges NOS
(C70.9)
Spinal cord (C72.0)
Cauda equina (C72.1)
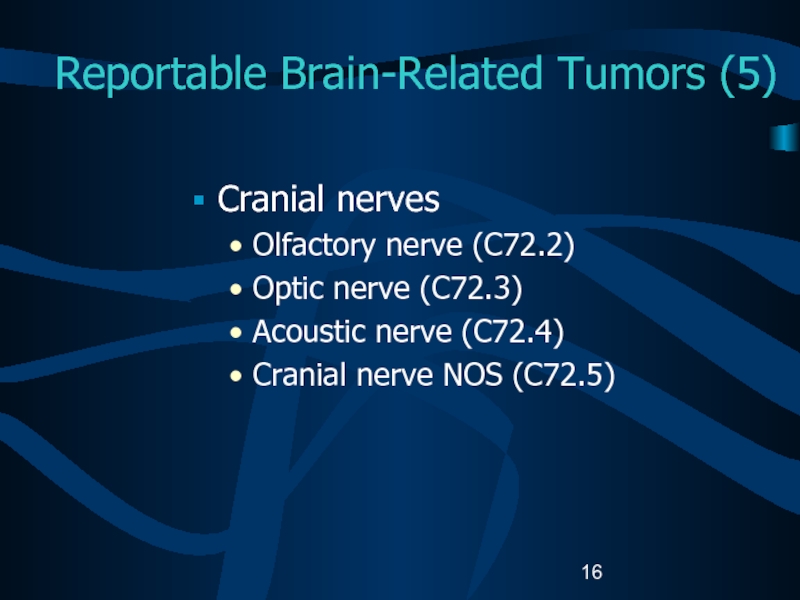
Слайд 16Reportable Brain-Related Tumors (5)
Cranial nerves
Olfactory nerve (C72.2)
Optic nerve (C72.3)
Acoustic nerve
(C72.4)
Cranial nerve NOS (C72.5)
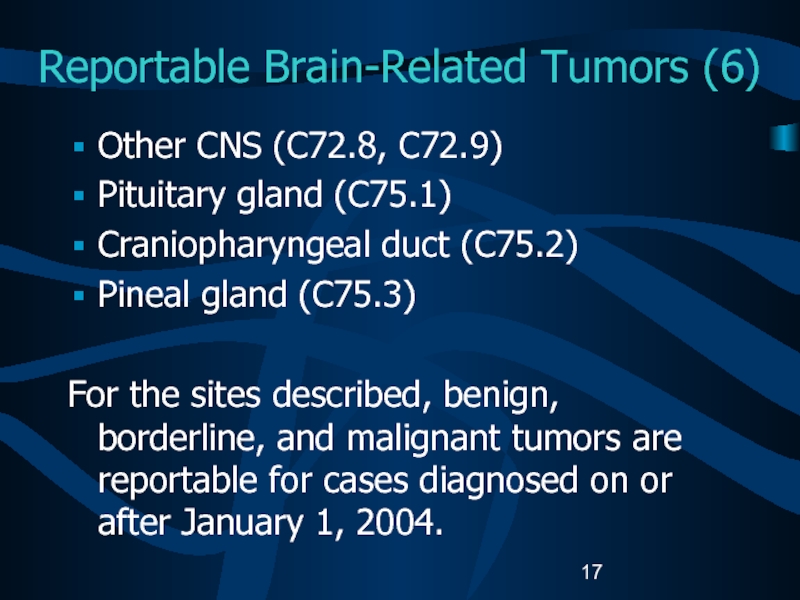
Слайд 17Reportable Brain-Related Tumors (6)
Other CNS (C72.8, C72.9)
Pituitary gland (C75.1)
Craniopharyngeal duct
(C75.2)
Pineal gland (C75.3)
For the sites described, benign, borderline, and malignant
tumors are reportable for cases diagnosed on or after January 1, 2004. Слайд 18History 2003
2003 SEER-supported registries and COC-approved hospital cancer registries will
also report non-malignant CNS tumors diagnosed on or after January
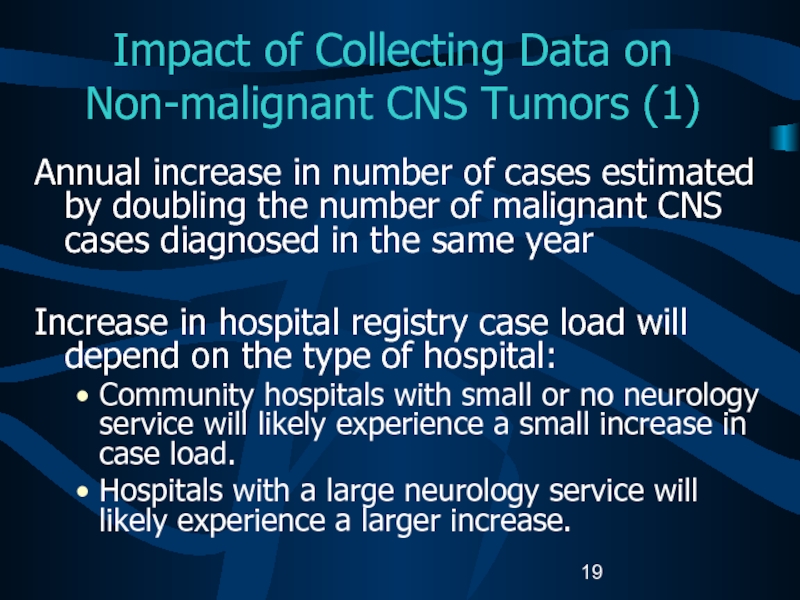
1, 2004.Слайд 19Impact of Collecting Data on Non-malignant CNS Tumors (1)
Annual increase
in number of cases estimated by doubling the number of
malignant CNS cases diagnosed in the same yearIncrease in hospital registry case load will depend on the type of hospital:
Community hospitals with small or no neurology service will likely experience a small increase in case load.
Hospitals with a large neurology service will likely experience a larger increase.
Слайд 20Impact of Collecting Data on Non-malignant CNS Tumors (2)
Central registry
case load is estimated to increase by 1%.
In 2002, 21
state cancer registries collected data on non-malignant CNS tumors: Minimal impact if registry’s definition for brain-related sites does not change.
Слайд 21Impact of Collecting Data on Non-malignant CNS Tumors (3)
Central registries
adding non-malignant CNS tumors to reportable case definition may have
to change state reporting law if law does not allow for collection of data on non-malignant cases.Слайд 22Impact of Collecting Data on Non-malignant CNS Tumors (4)
All cancer
registries must:
Have the same definition for brain-related tumors.
Implement data edits
created for non-malignant CNS tumors.Report rates for these tumors.
Слайд 23Case-finding (1)
Additional or expanded case-finding mechanisms:
Pathology
Radiology
Treatment facilities:
Radiation oncology centers
and departments
Gamma or cyber knife center.
Слайд 24Case-finding (2)
Disease indices
Surgery logs
Diagnostic imaging
Radiation oncology
Neurology clinics
Medical oncology
Autopsy reports.
Слайд 25Case-finding Sources
Free-standing radiation therapy centers
Free-standing Magnetic Resonance Imaging (MRI) centers
Free-standing
gamma or cyber knife centers
Free-standing oncology centers
Data exchange with other
central registriesDeath clearance process
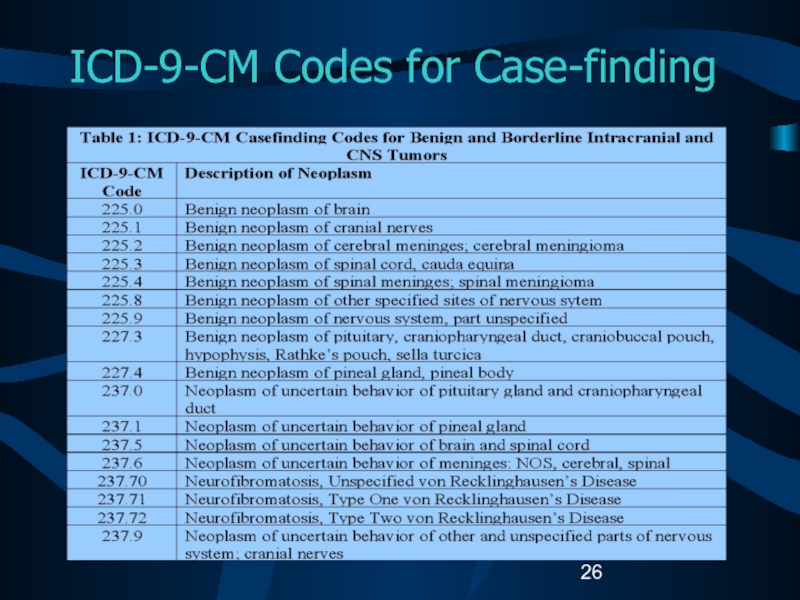
Слайд 27Unusual and Ambiguous Terminology
If the final pathologic diagnosis is a
CNS “neoplasm” or “mass”, an ICD-O-3 histology code must exist
for the case to be reportable.Hypodense mass or cystic neoplasm are not reportable, even for CNS sites.
A benign meningioma with a skull site should be coded to the cerebral meninges (C70.1).
Слайд 28Part II
CNS Anatomy and Function
Histologies and Primary Sites
Grading Systems and
Coding Grade
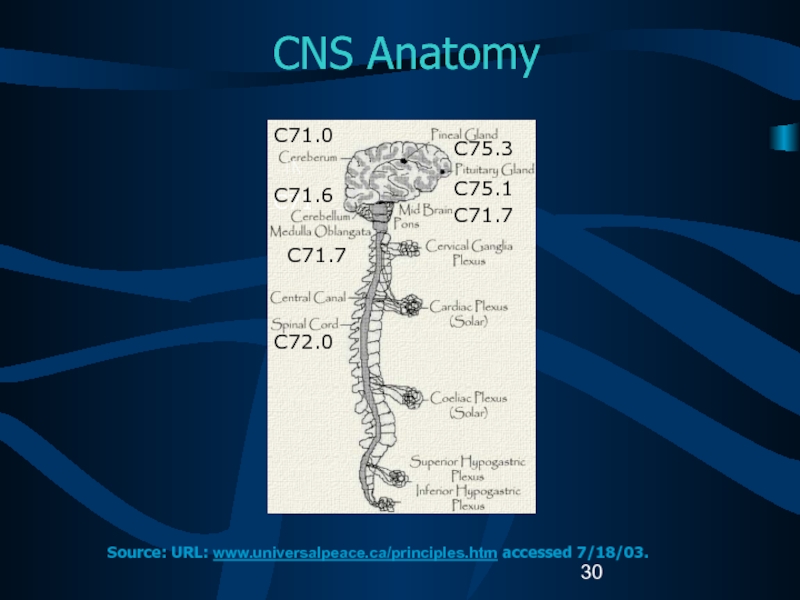
Слайд 30CNS Anatomy
C71
C71.6
C71.7
C72.0
C71.0
C75.3
C75.1
C71.7
Source: URL: www.universalpeace.ca/principles.htm accessed 7/18/03.
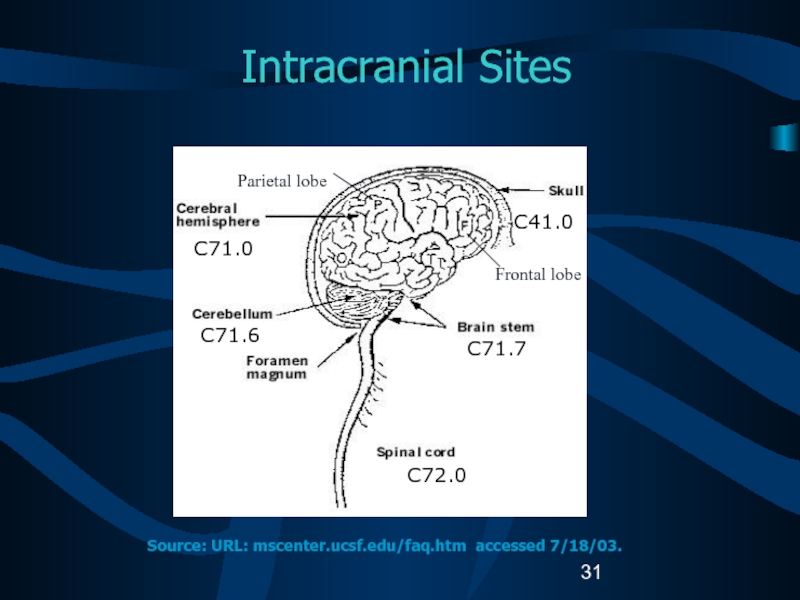
Слайд 31Intracranial Sites
C71.0
C71.6
C41.0
C71.7
C72.0
Source: URL: mscenter.ucsf.edu/faq.htm accessed 7/18/03.
Parietal lobe
Frontal lobe
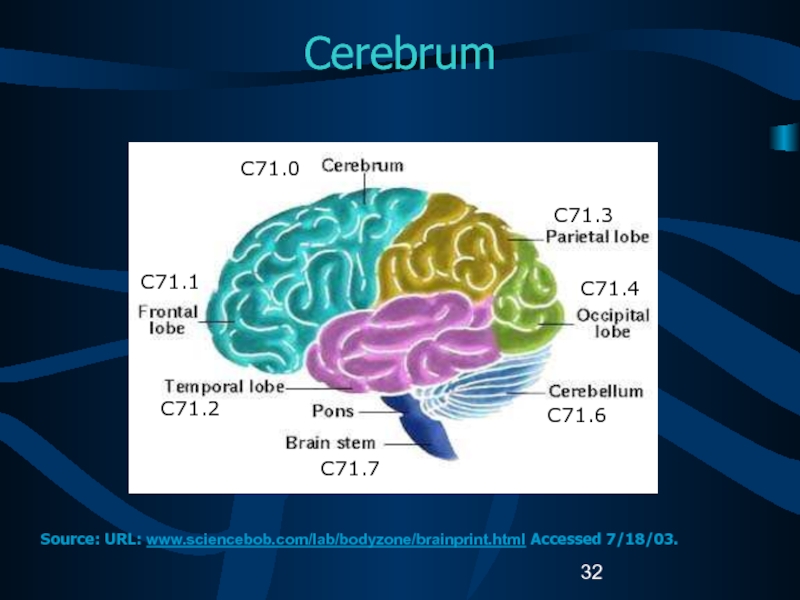
Слайд 32Cerebrum
C71.1
C71.2
C71.7
C71.3
C71.4
C71.6
C71.0
Source: URL: www.sciencebob.com/lab/bodyzone/brainprint.html Accessed 7/18/03.
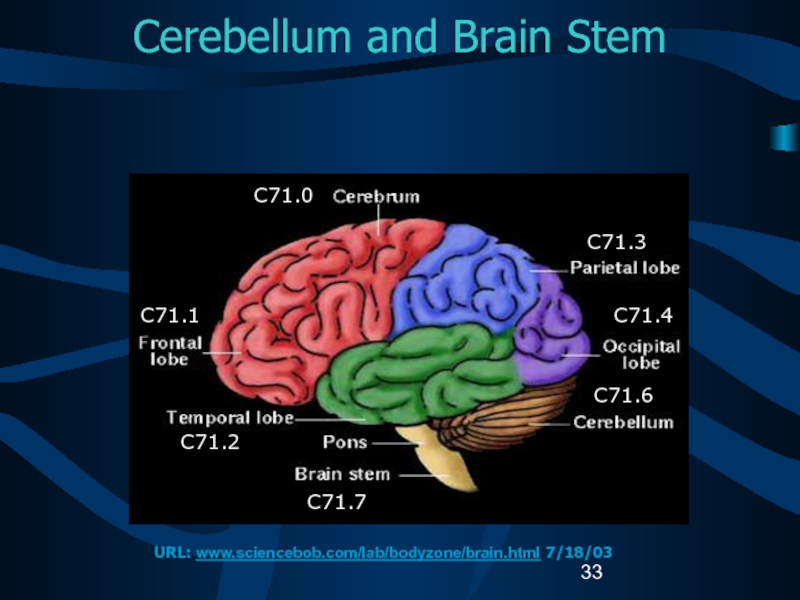
Слайд 33Cerebellum and Brain Stem
C71.0
C71.1
C71.2
C71.7
C71.3
C71.4
C71.6
URL: www.sciencebob.com/lab/bodyzone/brain.html 7/18/03
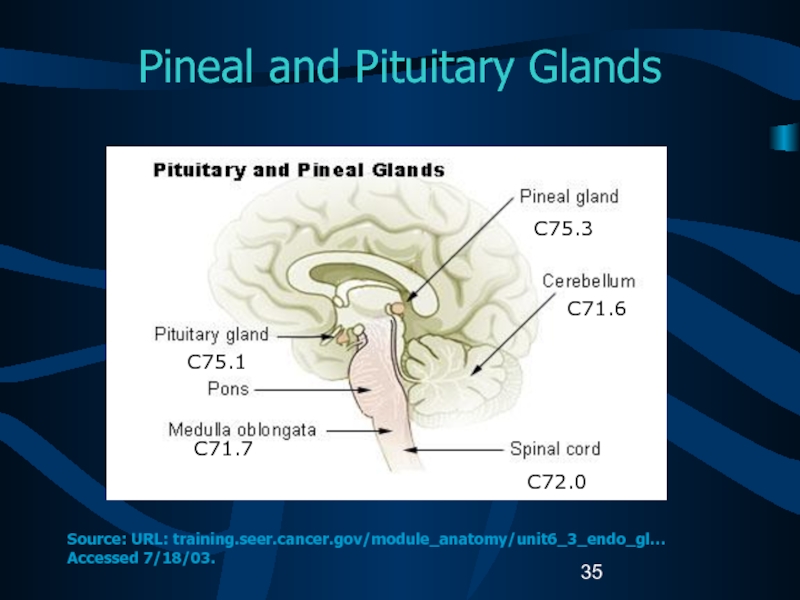
Слайд 35Pineal and Pituitary Glands
C75.1
C71.7
C75.3
C71.6
C72.0
Source: URL: training.seer.cancer.gov/module_anatomy/unit6_3_endo_gl… Accessed 7/18/03.
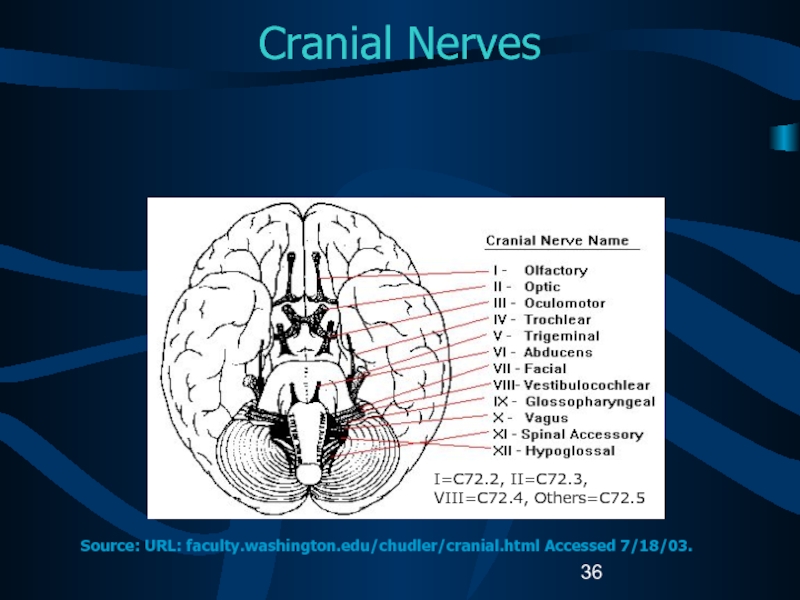
Слайд 36Cranial Nerves
I=C72.2, II=C72.3, VIII=C72.4, Others=C72.5
Source: URL: faculty.washington.edu/chudler/cranial.html Accessed 7/18/03.
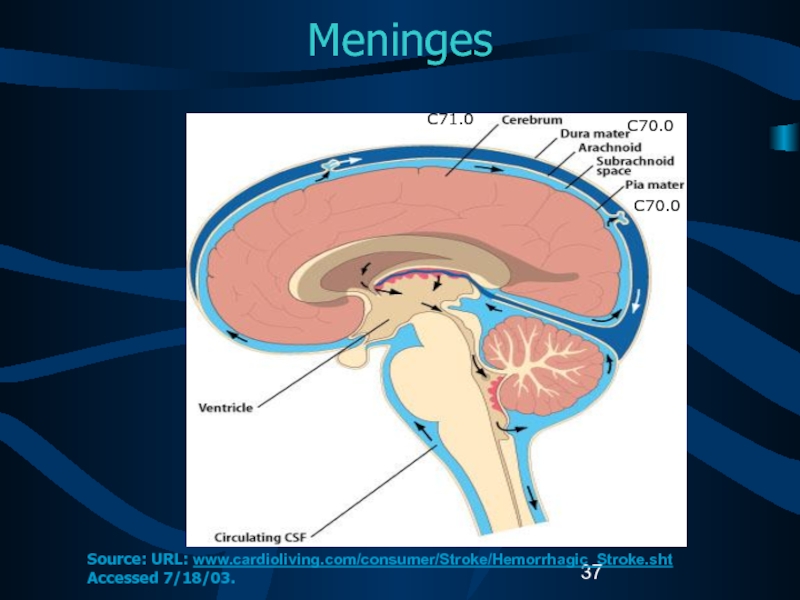
Слайд 37Meninges
C71.0
C70.0
C70.0
Source: URL: www.cardioliving.com/consumer/Stroke/Hemorrhagic_Stroke.sht Accessed 7/18/03.
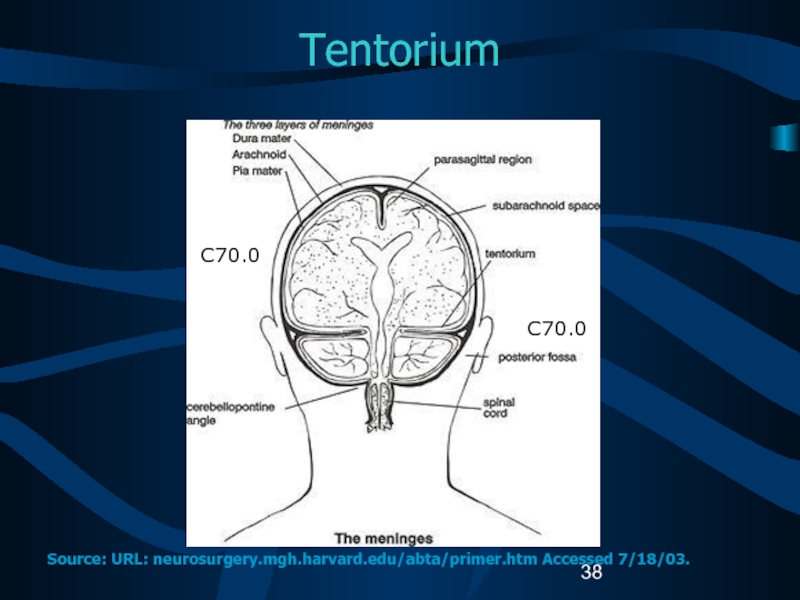
Слайд 38Tentorium
C70.0
C70.0
Source: URL: neurosurgery.mgh.harvard.edu/abta/primer.htm Accessed 7/18/03.
Слайд 39Spinal Cord
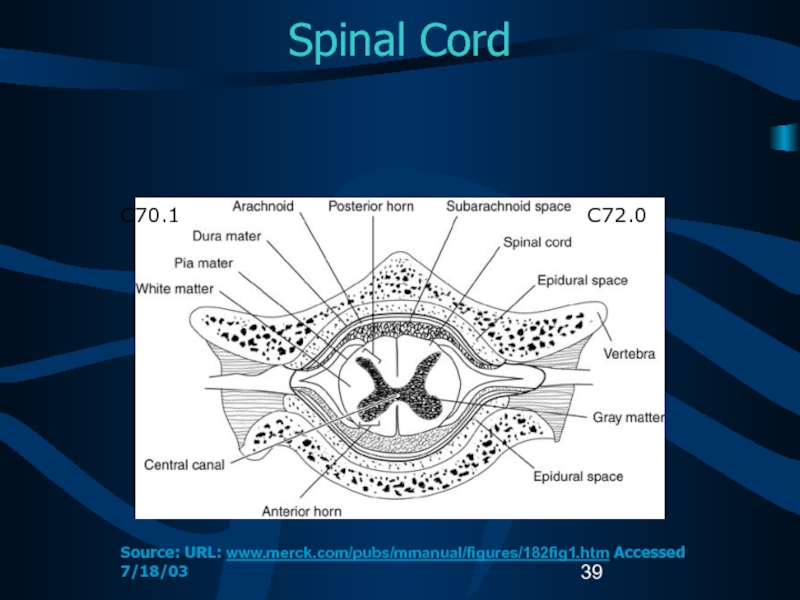
C72.0
C70.1
Source: URL: www.merck.com/pubs/mmanual/figures/182fig1.htm Accessed 7/18/03
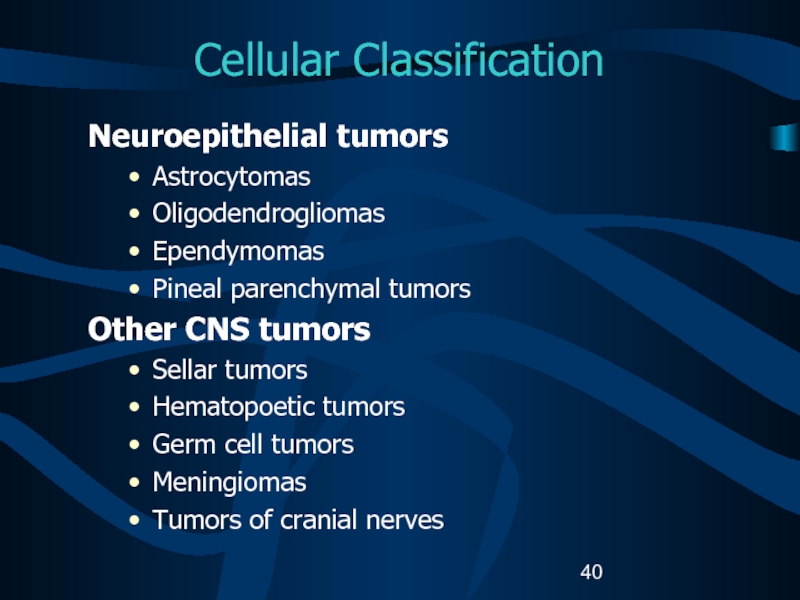
Слайд 40Cellular Classification
Neuroepithelial tumors
Astrocytomas
Oligodendrogliomas
Ependymomas
Pineal parenchymal tumors
Other CNS tumors
Sellar tumors
Hematopoetic
tumors
Germ cell tumors
Meningiomas
Tumors of cranial nerves
Слайд 41Glial Tumors (1)
Glial tissue: supportive tissue of brain made
up of astrocytes and oligodendrocytes
Glial tumors assigned ICD-O-3 histology codes
from glioma series:Codes 938 through 948.
Слайд 42Glial Tumors (2)
Astrocytic tumors
Noninfiltrating
Juvenile pilocytic (M9421)
Subependymal (M9383)
Infiltrating
Well-differentiated
mildly and moderately anaplastic astrocytomas (M9401)
Anaplastic astrocytomas
Glioblastoma multiforme
(M9440)Brain stem gliomas (M9380)
Слайд 43Glial Tumors (3)
Ependymal tumors
Myxopapillary and well-differentiated ependymomas (M9394)
Anaplastic ependymomas
(M9392)
Ependymoblastomas (M9392)
Oligodendroglial tumors
Well-differentiated oligodendrogliomas (M9450)
Anaplastic oligodendrogliomas (M9451)
Слайд 44Glial Tumors (4)
Mixed tumors
Mixed astrocytoma-ependymomas
Mixed astrocytoma-oligodendrogliomas
Mixed astrocytoma-ependymoma-oligodendrogliomas
Other
gliomas
Ganglioneuromas (M9490)
Optic nerve gliomas
Слайд 45Non-Glial Tumors (1)
Pineal region tumors
Parenchymal tumors
Pineocytomas (M9361)
Pineoblastomas (M9362)
Pineal astrocytomas
(M9400)
Germ cell tumors
Germinomas (M9064)
Embryonal carcinomas (M9070)
Choriocarcinomas (M9100)
Teratomas (M9080)
Слайд 46Non-Glial Tumors (2)
Meningiomas
Meningioma: Benign (M953_)
Malignant meningiomas
Anaplastic meningioma
Hemangiopericytoma (M9150)
Papillary meningioma (M9538)
Choroid
plexus tumors
Choroid plexus papilloma (M9390)
Choroid plexus carcinoma
Choroid plexus meningioma
(M9538)Слайд 47Other CNS Tumors (1)
Craniopharyngiomas (M9350)
Rathke pouch tumors
Chordomas (M9370)
Schwannomas (M9560)
Acoustic
schwannomas/neuromas
Слайд 48Other CNS Tumors (2)
Embryonal tumors
Retinoblastomas (M9510)
Primitive neuroectodermal tumors (PNETs)
Meduloblastomas (M9470)
Neuroblastomas (M9500)
Слайд 49Other CNS Tumors (3)
Lymphomas (M9590)
Arise from
Indigenous brain histiocytes (microglia)
Rare lymphocytes
in meninges
High incidence in patients with AIDS
Vascular tumors
Rare, non-malignant tumors
Arise
from blood vessels of brain and spinal cordHemangioblastoma (M9161) most common vascular tumor
Слайд 50Other CNS Tumors (4)
Cysts and tumor-like lesions
Reportable
Dermoid cysts
(M9084)
Granular cell tumors (M9580)
Rathke pouch tumors (M9350)
Not reportable
Epidermoid cysts
Colloid cysts
Enterogenous
cystsNeuroglial cysts
Plasma cell granulomas
Nasal glial herterotopias
Rathke cleft cysts
Слайд 51Childhood versus Adult Tumors
CNS tumor histology and location are different
in adult and children.
Tumor location and extent of spread affect
treatment and prognosis.Most common solid tumor in childhood.
Слайд 52Childhood Brain Tumors
Meduloblastomas are the most common CNS histology in
children.
50% are infratentorial.
Common infratentorial tumors:
Cerebellar astrocytomas
Meduloblastomas
Ependymomas
Brain stem gliomas
Atypical
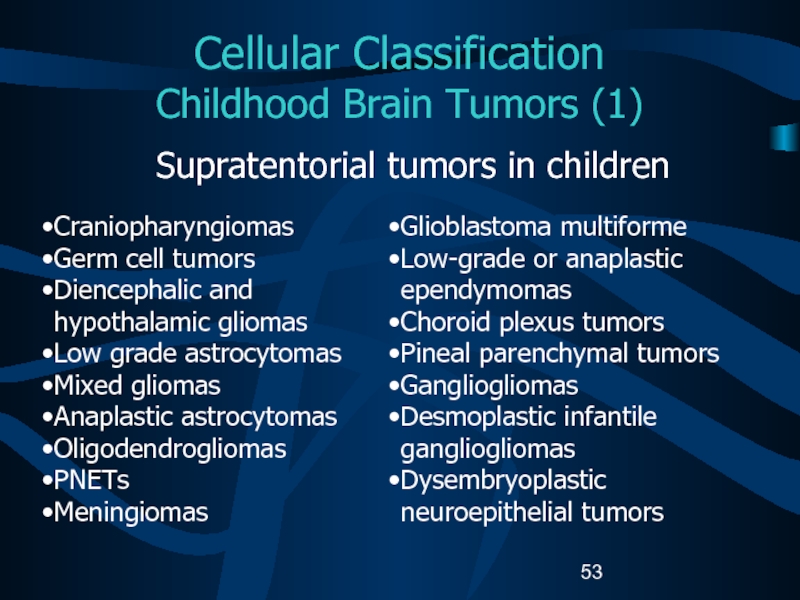
teratoid tumorsСлайд 53Cellular Classification
Childhood Brain Tumors (1)
Supratentorial tumors in children
Craniopharyngiomas
Germ cell
tumors
Diencephalic and hypothalamic gliomas
Low grade astrocytomas
Mixed gliomas
Anaplastic astrocytomas
Oligodendrogliomas
PNETs
Meningiomas
Glioblastoma
multiformeLow-grade or anaplastic ependymomas
Choroid plexus tumors
Pineal parenchymal tumors
Gangliogliomas
Desmoplastic infantile gangliogliomas
Dysembryoplastic neuroepithelial tumors
Слайд 54Cellular Classification
Childhood Brain Tumors (2)
The histopathology of childhood spinal
tumors is the same as for childhood brain tumors.
Primary spinal
cord tumors comprise approximately 1% to 2% of all childhood CNS tumors.Слайд 55Cellular Classification
Childhood CNS Tumors
Cause of childhood CNS tumors remains
unknown.
American Academy of Pediatrics has outlined guidelines for pediatric cancer
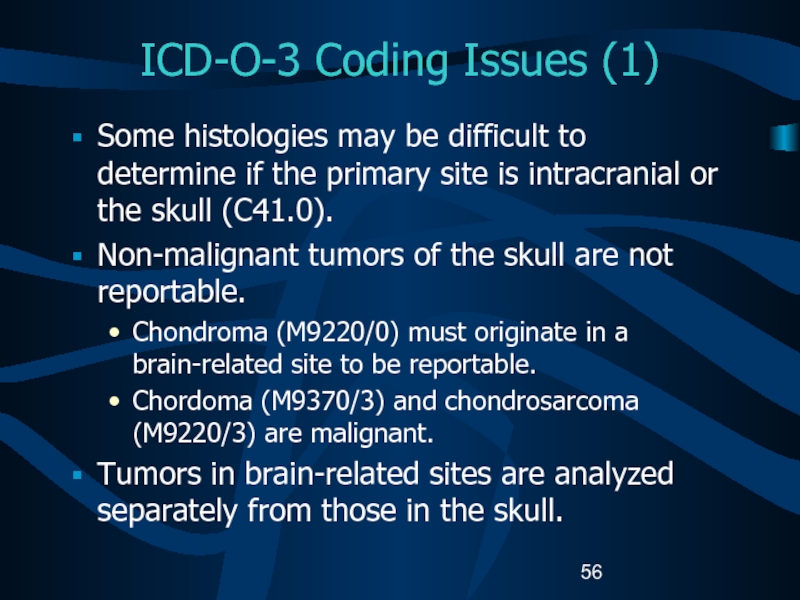
centers and their role in the treatment of pediatric cancer patients.Слайд 56ICD-O-3 Coding Issues (1)
Some histologies may be difficult to determine
if the primary site is intracranial or the skull (C41.0).
Non-malignant
tumors of the skull are not reportable.Chondroma (M9220/0) must originate in a brain-related site to be reportable.
Chordoma (M9370/3) and chondrosarcoma (M9220/3) are malignant.
Tumors in brain-related sites are analyzed separately from those in the skull.
Слайд 57ICD-O-3 Coding Issues (2)
Continue to assign histology code M9421/3 to
pilocytic astrocytoma.
When the primary site for intracranial schwannoma (9560/0)
is not documented in source documents, the site should be coded to cranial nerves NOS (C72.5).Слайд 58Grade for CNS Tumors
Sixth digit of ICD-O-3 histology code
Describes
tumor differentiation or grade.
Is not usually specified for CNS tumors.
Is always assigned code 9 for non-malignant CNS tumors:
Not determined, not stated, or not applicable.
Per ICD-O-3, page 30, Rule G, paragraph 1 “Only malignant tumors are graded.”
Not the same as WHO grade.
Слайд 59WHO Grade (1)
WHO grade coded in Collaborative Stage data field:
Site-specific factor 1 for Brain.
Four-category tumor grading system
Grade I
Slow
growing Non-malignant tumors
Patients have long-term survival.
Слайд 60WHO Grade (2)
Grade II
Relatively slow growing
Sometimes recur as higher
grade tumors
May be non-malignant or malignant .
Grade III
Malignant tumors
Often
recur as higher grade tumors.Grade IV
Highly malignant and aggressive.
Слайд 61Kernohan Grade
Defines progressive malignancy for astrocytoma
Grade 1: benign astrocytomas
Grade 2:
low-grade astrocytomas
Grade 3: anaplastic astrocytomas
Grade 4: glioblastoma multiforme
No NAACCR data
field for Kernohan grade.Слайд 62St. Anne-Mayo Grade (1)
Used for astrocytomas.
Uses four morphologic criteria:
Nuclear
atypia
Mitosis
Endothelial proliferation
Necrosis
No NAACCR data field for the St. Anne-Mayo grade.
Слайд 63St. Anne-Mayo Grade (2)
Grade 1: No criteria
Grade 2: One criterion,
usually nuclear atypia
Grade 3: Two criteria, usually nuclear atypia and
mitosisGrade 4: Three or four criteria
Слайд 64Grade for CNS Tumors
Do not record WHO grade, Kernohan grade,
or St. Anne/Mayo grade in the sixth digit histology code
data fieldСлайд 65Part III
Laterality
Multiple Primaries
Malignant Transformation
Sequence Numbers
Date of Diagnosis
Слайд 66Determining Multiple Primaries:
Laterality
Brain is not a paired organ.
Laterality collected
on both non-malignant and malignant tumors.
Used to determine if multiple
non-malignant CNS tumors are counted as multiple primary tumors.Not used to determine if multiple malignant tumors of the same intracranial or CNS site are multiple primary tumors.
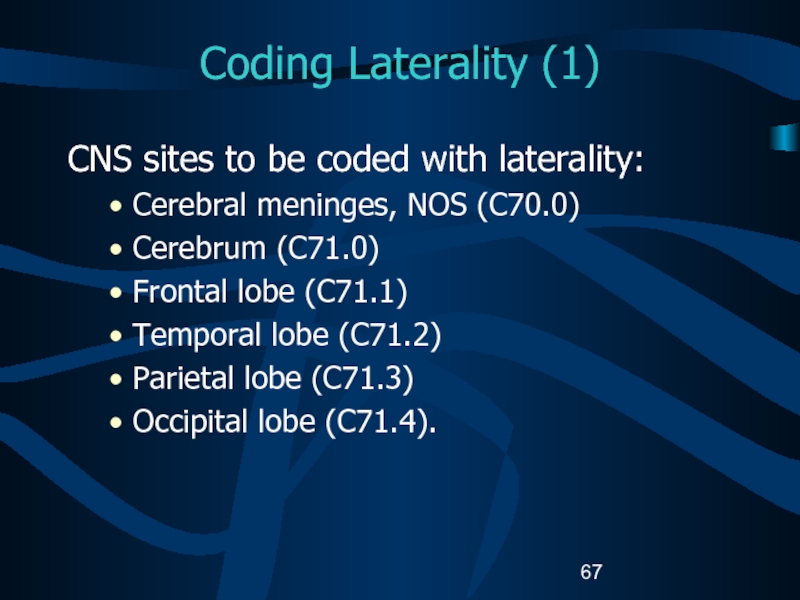
Слайд 67Coding Laterality (1)
CNS sites to be coded with laterality:
Cerebral meninges,
NOS (C70.0)
Cerebrum (C71.0)
Frontal lobe (C71.1)
Temporal lobe (C71.2)
Parietal lobe (C71.3)
Occipital lobe
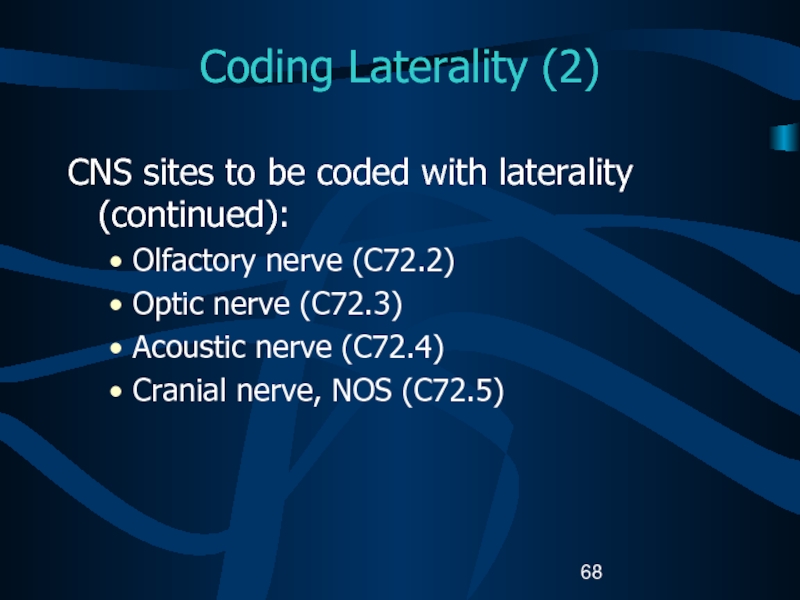
(C71.4).Слайд 68Coding Laterality (2)
CNS sites to be coded with laterality (continued):
Olfactory
nerve (C72.2)
Optic nerve (C72.3)
Acoustic nerve (C72.4)
Cranial nerve, NOS (C72.5)
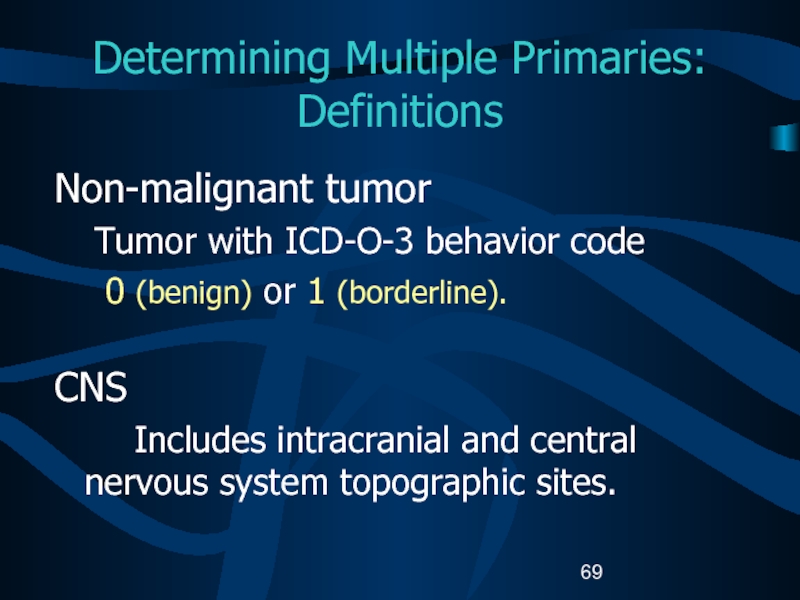
Слайд 69Determining Multiple Primaries:
Definitions
Non-malignant tumor
Tumor with ICD-O-3 behavior code
0 (benign)
or 1 (borderline).
CNS
Includes intracranial and central nervous system topographic sites.
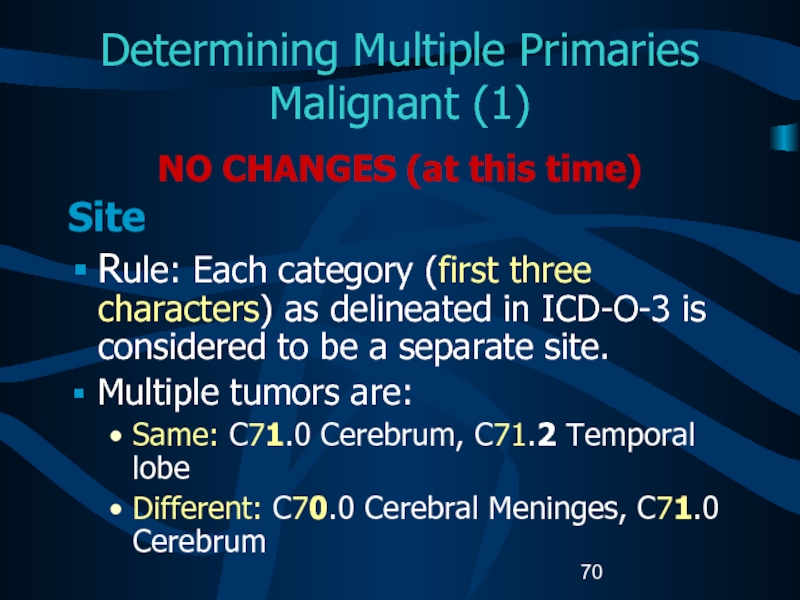
Слайд 70Determining Multiple Primaries
Malignant (1)
NO CHANGES (at this time)
Site
Rule: Each
category (first three characters) as delineated in ICD-O-3 is considered
to be a separate site.Multiple tumors are:
Same: C71.0 Cerebrum, C71.2 Temporal lobe
Different: C70.0 Cerebral Meninges, C71.0 Cerebrum
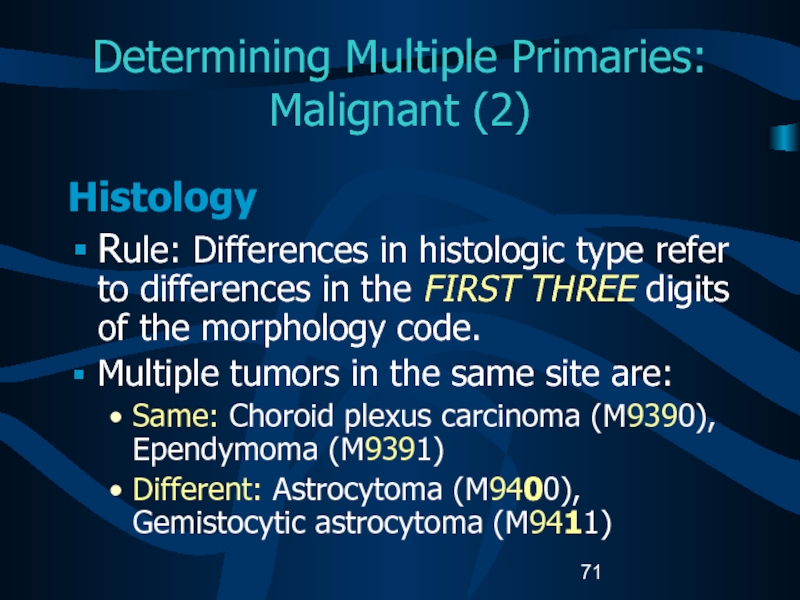
Слайд 71Determining Multiple Primaries:
Malignant (2)
Histology
Rule: Differences in histologic type
refer to differences in the FIRST THREE digits of the
morphology code.Multiple tumors in the same site are:
Same: Choroid plexus carcinoma (M9390), Ependymoma (M9391)
Different: Astrocytoma (M9400), Gemistocytic astrocytoma (M9411)
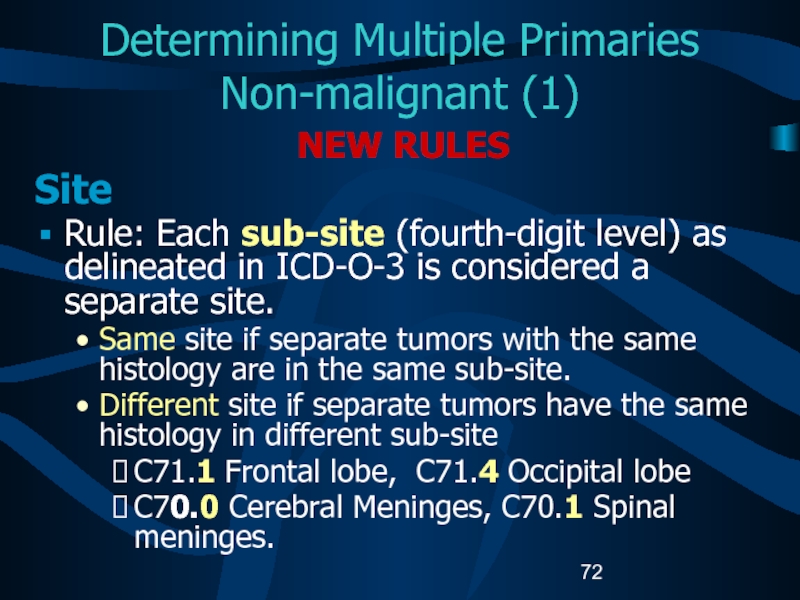
Слайд 72Determining Multiple Primaries
Non-malignant (1)
NEW RULES
Site
Rule: Each sub-site (fourth-digit level)
as delineated in ICD-O-3 is considered a separate site.
Same site
if separate tumors with the same histology are in the same sub-site.Different site if separate tumors have the same histology in different sub-site
C71.1 Frontal lobe, C71.4 Occipital lobe
C70.0 Cerebral Meninges, C70.1 Spinal meninges.
Слайд 73Determining Multiple Primaries
Non-malignant (2)
Site (cont)
EXCEPT NOS (C_ _.9) with
specific four-digit site code in same rubric
Example: meninges, NOS
(C70.9) with spinal meninges (C70.1) or cerebral meninges (C70.0).Слайд 74Determining Multiple Primaries
Non-malignant (3)
Site (cont)
Laterality: For non-malignant cases only
If
multiple tumors of the same site and same histologic type
are identified and both sides of a site listed as lateral are involved, tumors should be counted as separate primaries.Different:
Right temporal lobe (C71.2) and left temporal lobe (C71.2)
Слайд 76Determining Multiple Primaries:
Non-malignant (5)
Histology
If multiple tumors are in the
same site, refer to Table 2, and use the following
rules in priority order:A-1: If the first three digits are the same but the codes are not found in Table 2, then the histology is considered to be the SAME.
A-2: If the first three digits are different but the codes are not found in Table 2, then the histology is considered to be DIFFERENT.
Слайд 77Determining Multiple Primaries:
Non-malignant (6)
Histology (cont.)
B. If all histologies are
listed in the same histologic group in Table 2, then
the histology is considered to be the SAME. *Example: Ependymomas: M9394, Myxopapillary ependymoma and M9444, Chordoid glioma have the same histology
*Note: If two histologies are in the same group in Table 2, code the first or more specific histology.
Слайд 78Determining Multiple Primaries:
Non-malignant (7)
Histology (cont)
C: If the first three
digits are the same as the first three digits for
any histology in one of the groupings in Table 2 , then the histology is considered to be the SAME.*Example: On table: Neuronal and neurol-glial neoplasm: M9505, ganglioglioma, Not on table: M9507, Pacinian tumor
* Note: If two histologies are in the same group in Table 2, code the first or more specific histology.
Слайд 79Determining Multiple Primaries:
Non-malignant (8)
Histology (cont)
D: If the first three
digits are the same and the histologies are from two
different groups in the histologic groupings table, the histologies are considered to be DIFFERENT.Example: Gliomas: M9442, Gliofibroma; Ependymoma: M9444, Chordoid glioma
Слайд 80Determining Multiple Primaries:
Timing (1)
Primary malignant CNS tumors
NO CHANGE
Malignant tumors
of the same site and same histology, diagnosed within 2
months:Tumors are counted as the SAME primary.
Malignant tumors of the same site and same histology, diagnosed more than 2 months apart:
Tumors are counted as DIFFERENT primary sites.
Слайд 81Determining Multiple Primaries:
Timing (2)
Primary non-malignant CNS tumors
NEW
No timing rule
If a
new non-malignant tumor of the same histology as an earlier
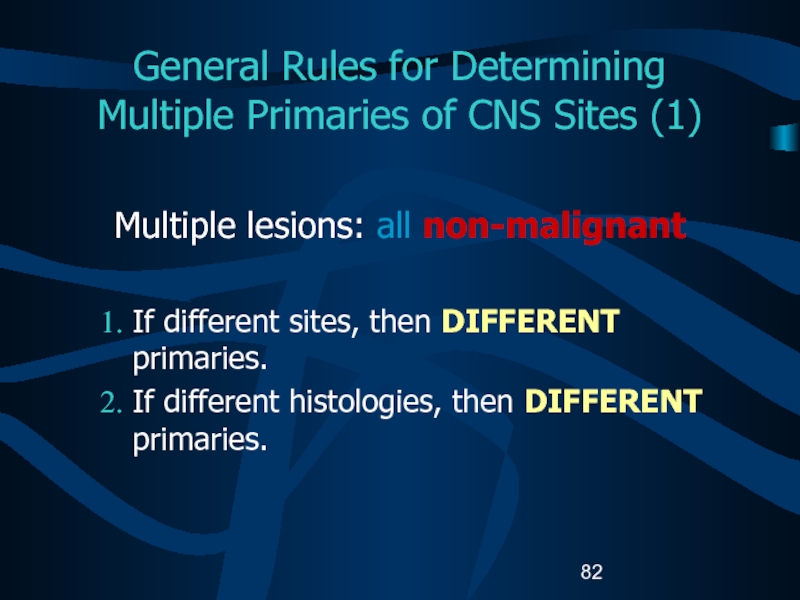
tumor that had been diagnosed in the same site is diagnosed subsequently at any time, it is considered to be the SAME primary tumor.Слайд 82General Rules for Determining Multiple Primaries of CNS Sites (1)
Multiple
lesions: all non-malignant
If different sites, then DIFFERENT primaries.
If different histologies,
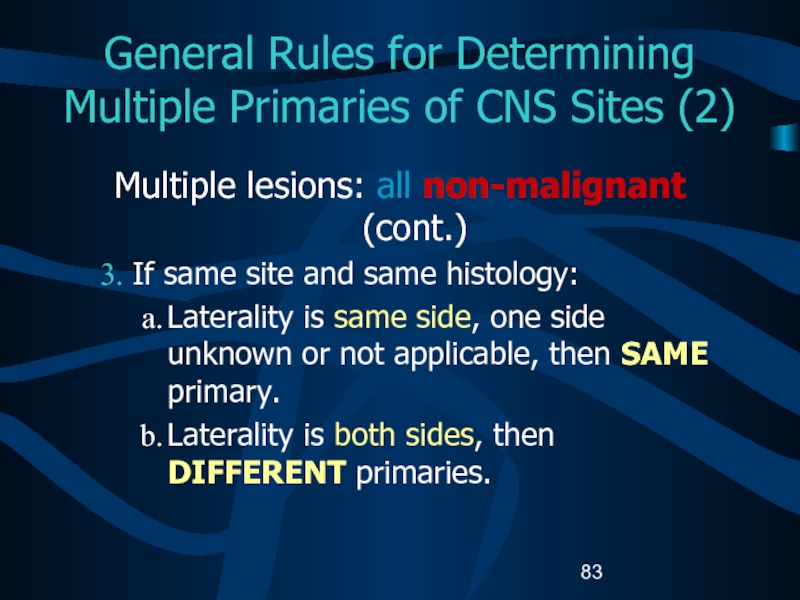
then DIFFERENT primaries.Слайд 83General Rules for Determining Multiple Primaries of CNS Sites (2)
Multiple
lesions: all non-malignant (cont.)
If same site and same histology:
Laterality is
same side, one side unknown or not applicable, then SAME primary.Laterality is both sides, then DIFFERENT primaries.
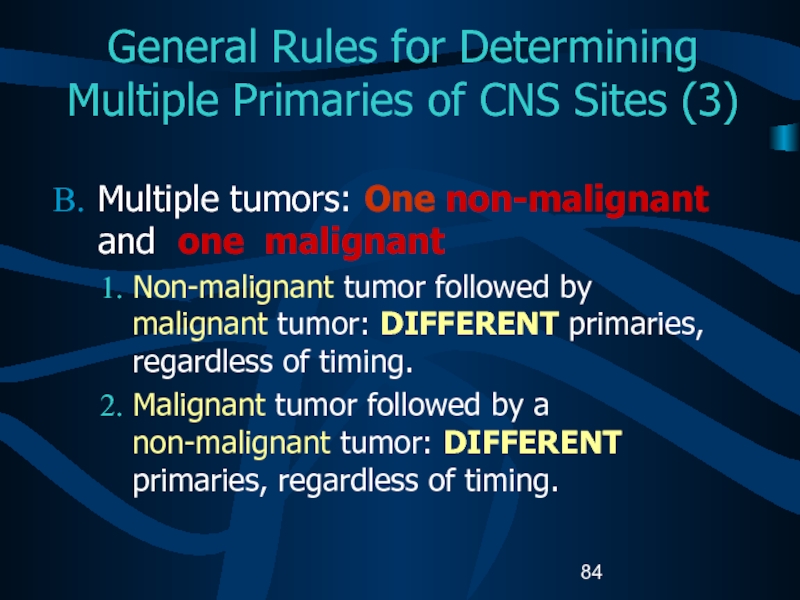
Слайд 84General Rules for Determining Multiple Primaries of CNS Sites (3)
Multiple
tumors: One non-malignant and one malignant
Non-malignant tumor followed by
malignant tumor: DIFFERENT primaries, regardless of timing.Malignant tumor followed by a non-malignant tumor: DIFFERENT primaries, regardless of timing.
Слайд 85Histologic Transformation (1)
Histologic transformation or progression to a higher grade:
Determined
by pathological review.
Final diagnosis made by review of previous
biopsies or excisions and comparison to newly biopsied or resected brain tumorNon-malignant tumor transforms to malignant tumor.
Malignant tumors transforms to higher grade tumor.
Слайд 86Histologic Transformation (2)
If a malignant CNS tumor recurs (transforms) as
a higher grade tumor,
SAME tumor.
Do not change the histology or
grade.Do not abstract as new primary.
Example: Astrocytoma (M9400) transforms to glioblastoma multiforme (M9440).
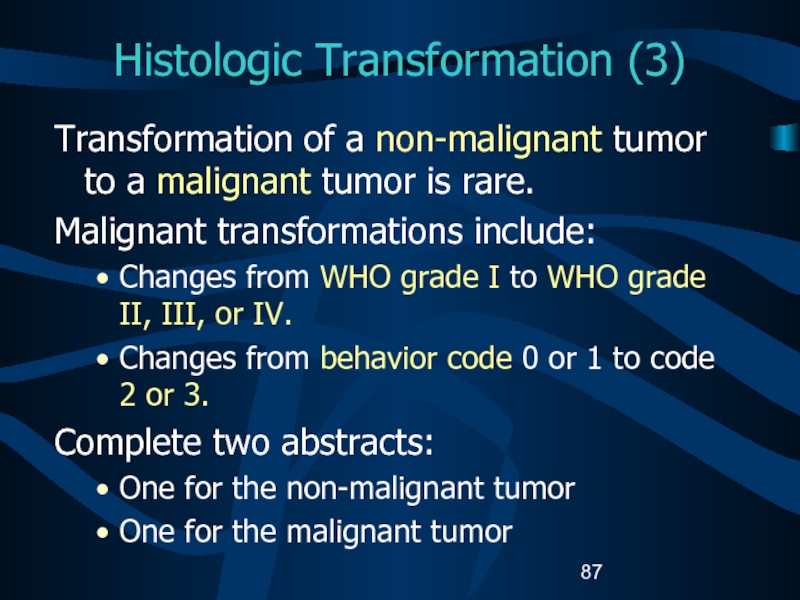
Слайд 87Histologic Transformation (3)
Transformation of a non-malignant tumor to a malignant
tumor is rare.
Malignant transformations include:
Changes from WHO grade I to
WHO grade II, III, or IV.Changes from behavior code 0 or 1 to code 2 or 3.
Complete two abstracts:
One for the non-malignant tumor
One for the malignant tumor
Слайд 88Histologic Transformation (4)
Sequence Numbers
Non-malignant tumors: assigned sequence numbers from the
reportable-by-agreement series.
Malignant tumors: assigned sequence numbers from the malignant series.
Example:
Patient has a non-malignant CNS tumor that progressed into a malignant CNS tumor:Non-malignant tumor is sequenced as 60.
Malignant tumor is sequenced as 00.
Слайд 89Histologic Transformation (5)
Date of Diagnosis
Non-malignant tumors: First date that a
medical practitioner diagnosed the non-malignant tumor either clinically or histologically.
Malignant
tumors: First date that a medical practitioner diagnosed the malignant transformation either clinically or histologically.Слайд 90Coding Sequence Numbers (1)
Indicates the sequence of all reportable neoplasms
over the lifetime of the person.
Codes 00 – 35: Malignant
and in situ reportable neoplasms. Codes 60 – 87: Reportable-by-agreement including non-malignant tumors diagnosed after January1, 2004.
Слайд 91Coding Sequence Numbers (2)
Reportable-by-agreement neoplasms are defined by each facility
and/or central cancer registry:
Non-malignant CNS tumors are assigned reportable-by-agreement sequence
numbers even when they are reportable.Codes 60 – 87
Слайд 92Coding Sequence Numbers (3)
Sequence numbers for non-malignant CNS tumors are
assigned over the lifetime of the person.
Example: Patient diagnosed with
a non-malignant CNS tumor in January, 2003 (not reportable by state or hospital reporting rules) and diagnosed with second non-malignant CNS tumor in 2004: Second is sequence number 62.
Complete abstract for the second tumor only.
Слайд 93Assigning Diagnosis Date
Rules for assigning diagnosis date are the same
for malignant and non-malignant tumors.
Review source records carefully to
determine initial diagnosis date, regardless of whether it is a clinical or histological diagnosis.Слайд 95Collaborative Stage (CS)
A computer algorithm uses the collaborative stage (CS)
data fields to calculate site-specific American Joint Committee on Cancer
(AJCC) TNM stage, SEER Summary Stage 1977, and SEER Summary Stage 2000.Слайд 96Coding Collaborative Stage (1)
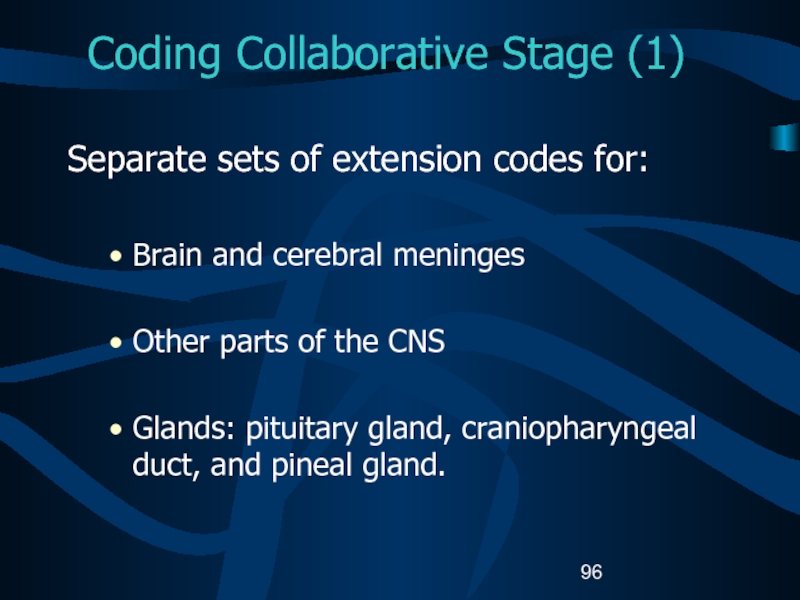
Separate sets of extension codes for:
Brain and
cerebral meninges
Other parts of the CNS
Glands: pituitary gland, craniopharyngeal duct,
and pineal gland.Слайд 97Coding Collaborative Stage (2)
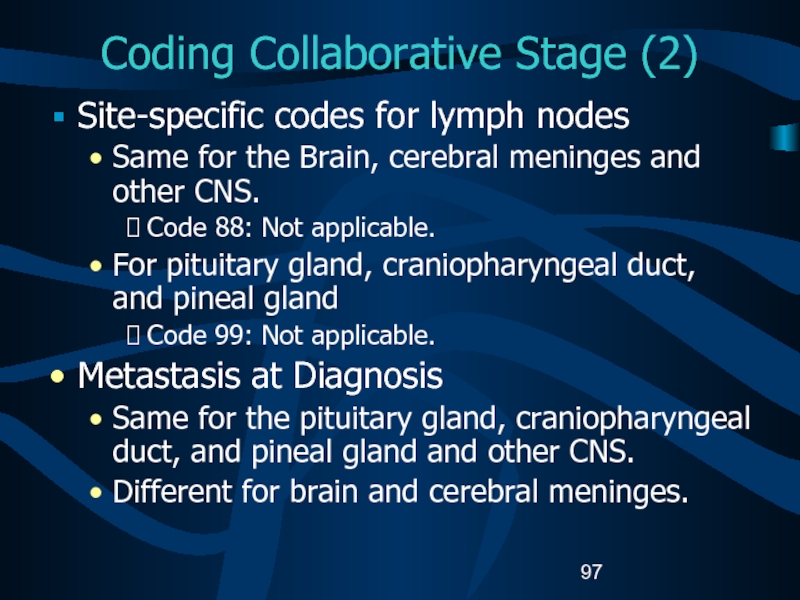
Site-specific codes for lymph nodes
Same for
the Brain, cerebral meninges and other CNS.
Code 88: Not applicable.
For
pituitary gland, craniopharyngeal duct, and pineal glandCode 99: Not applicable.
Metastasis at Diagnosis
Same for the pituitary gland, craniopharyngeal duct, and pineal gland and other CNS.
Different for brain and cerebral meninges.
Слайд 98CS Extension: Brain and Meninges
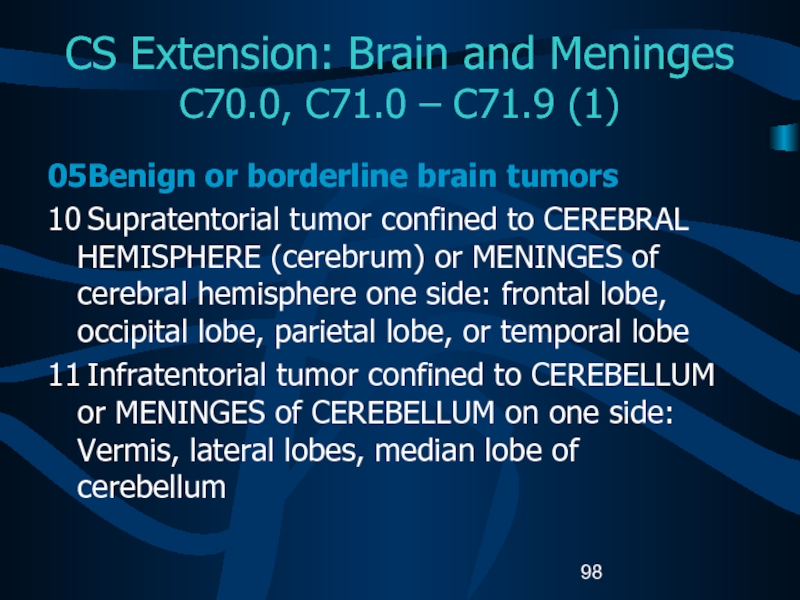
C70.0, C71.0 – C71.9 (1)
05 Benign or
borderline brain tumors
10 Supratentorial tumor confined to CEREBRAL HEMISPHERE (cerebrum) or
MENINGES of cerebral hemisphere one side: frontal lobe, occipital lobe, parietal lobe, or temporal lobe11 Infratentorial tumor confined to CEREBELLUM or MENINGES of CEREBELLUM on one side: Vermis, lateral lobes, median lobe of cerebellum
Слайд 99CS Extension: Brain and Meninges
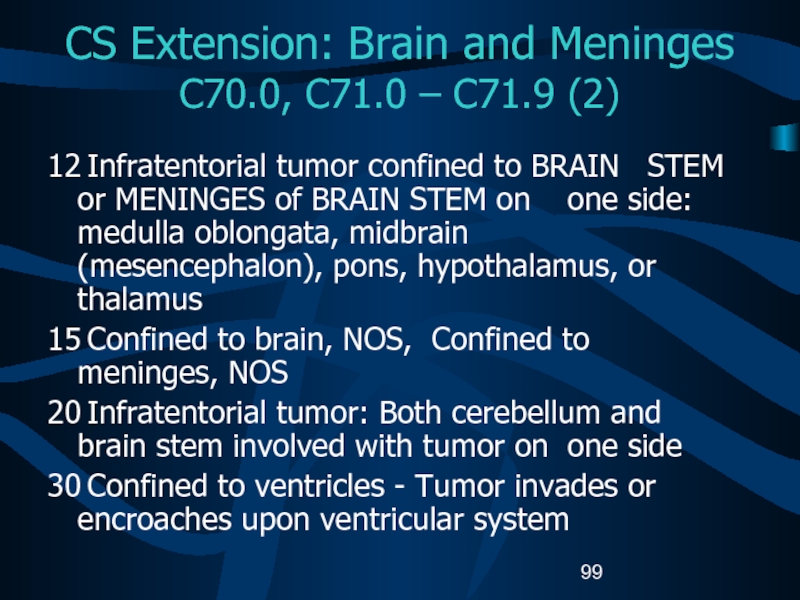
C70.0, C71.0 – C71.9 (2)
12 Infratentorial
tumor confined to BRAIN STEM or MENINGES of BRAIN STEM
on one side: medulla oblongata, midbrain (mesencephalon), pons, hypothalamus, or thalamus15 Confined to brain, NOS, Confined to meninges, NOS
20 Infratentorial tumor: Both cerebellum and brain stem involved with tumor on one side
30 Confined to ventricles - Tumor invades or encroaches upon ventricular system
Слайд 100CS Extension: Brain and Meninges
C70.0, C71.0 – C71.9 (3)
40 Tumor crosses
the midline: involves the contralateral hemisphere, involves corpus callosum (including
splenium)50 Supratentorial tumor extends infratentorially to involve cerebellum or brain stem
51 Infratentorial tumor extends supratentorially to involve cerebrum (cerebral hemisphere)
60 Tumor invades bone (skull), major blood vessel(s), meninges (dura), nerves, NOS (cranial nerves), or spinal cord/canal
Слайд 101CS Extension: Brain and Meninges
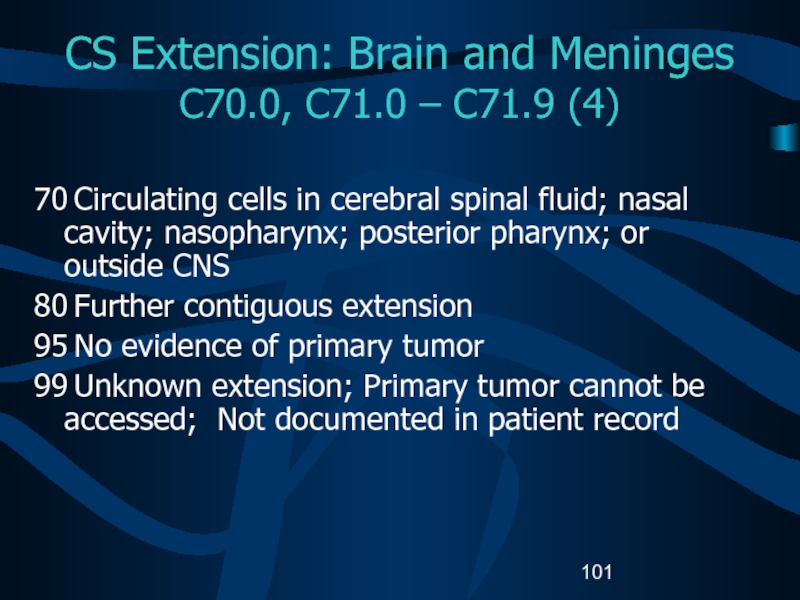
C70.0, C71.0 – C71.9 (4)
70 Circulating cells
in cerebral spinal fluid; nasal cavity; nasopharynx; posterior pharynx; or
outside CNS80 Further contiguous extension
95 No evidence of primary tumor
99 Unknown extension; Primary tumor cannot be accessed; Not documented in patient record
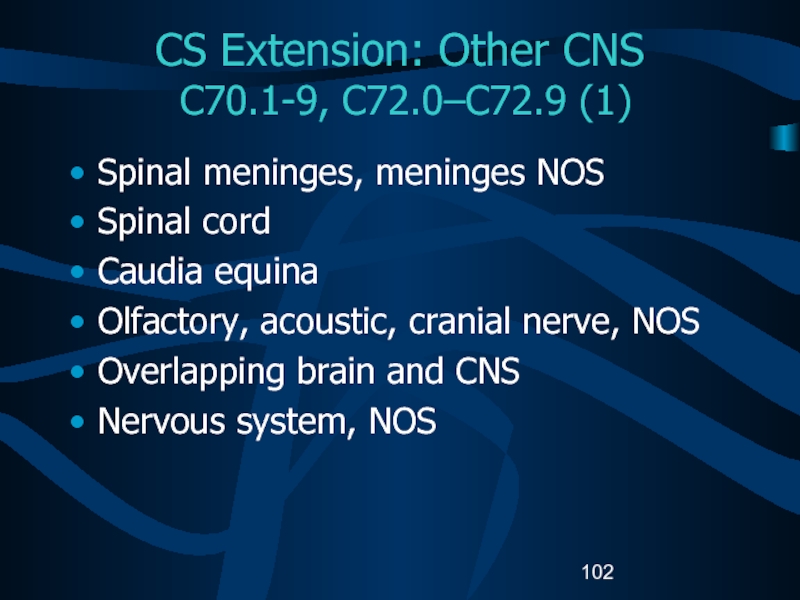
Слайд 102CS Extension: Other CNS
C70.1-9, C72.0–C72.9 (1)
Spinal meninges, meninges NOS
Spinal
cord
Caudia equina
Olfactory, acoustic, cranial nerve, NOS
Overlapping brain and CNS
Nervous system,
NOSСлайд 103CS Extension: Other CNS
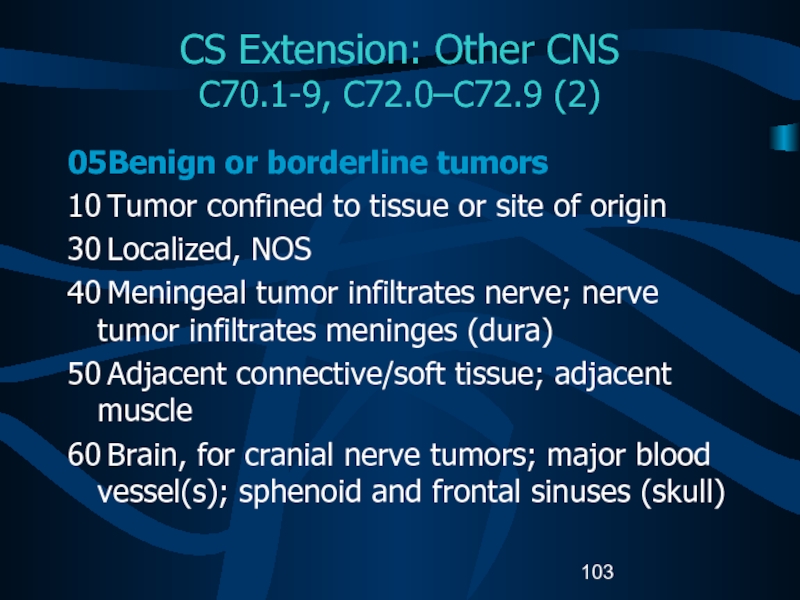
C70.1-9, C72.0–C72.9 (2)
05 Benign or borderline tumors
10 Tumor confined
to tissue or site of origin
30 Localized, NOS
40 Meningeal tumor infiltrates nerve;
nerve tumor infiltrates meninges (dura)50 Adjacent connective/soft tissue; adjacent muscle
60 Brain, for cranial nerve tumors; major blood vessel(s); sphenoid and frontal sinuses (skull)
Слайд 104CS Extension: Other CNS
C70.1-9, C72.0–C72.9 (3)
70 Brain except for cranial
nerve tumors; bone, other than skull; eye
80 Further contiguous extension
95 No evidence
of primary tumor99 Unknown extension; primary tumor cannot be assessed; not documented in patient record
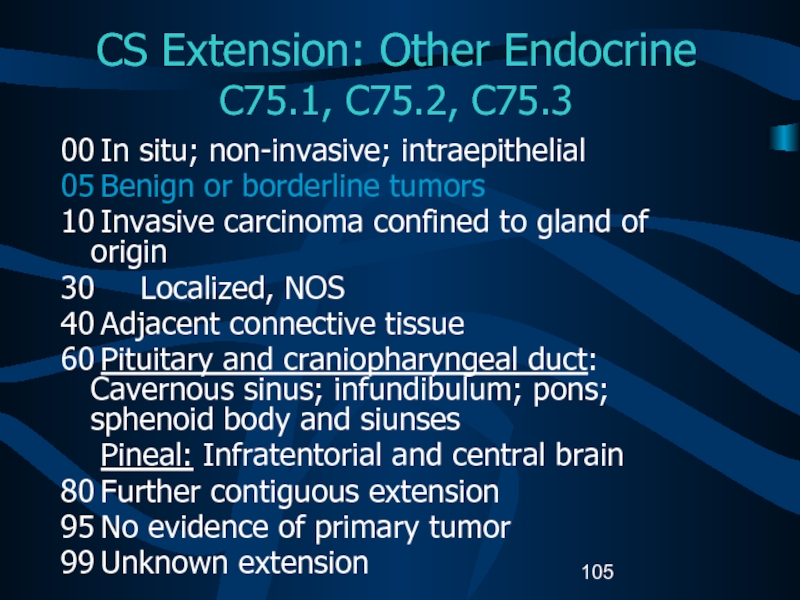
Слайд 105CS Extension: Other Endocrine
C75.1, C75.2, C75.3
00 In situ; non-invasive; intraepithelial
05 Benign
or borderline tumors
10 Invasive carcinoma confined to gland of origin
30 Localized,
NOS40 Adjacent connective tissue
60 Pituitary and craniopharyngeal duct: Cavernous sinus; infundibulum; pons; sphenoid body and siunses
Pineal: Infratentorial and central brain
80 Further contiguous extension
95 No evidence of primary tumor
99 Unknown extension
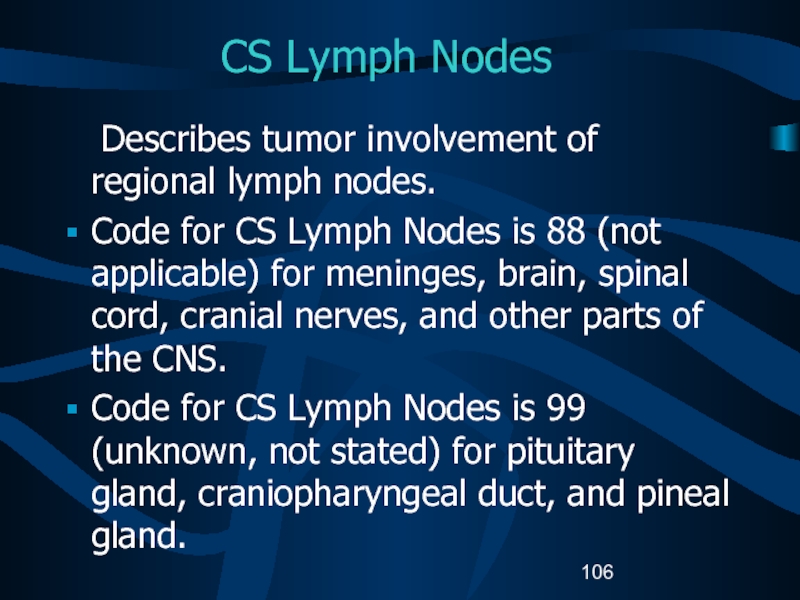
Слайд 106CS Lymph Nodes
Describes tumor involvement of regional lymph nodes.
Code for
CS Lymph Nodes is 88 (not applicable) for meninges, brain,
spinal cord, cranial nerves, and other parts of the CNS.Code for CS Lymph Nodes is 99 (unknown, not stated) for pituitary gland, craniopharyngeal duct, and pineal gland.
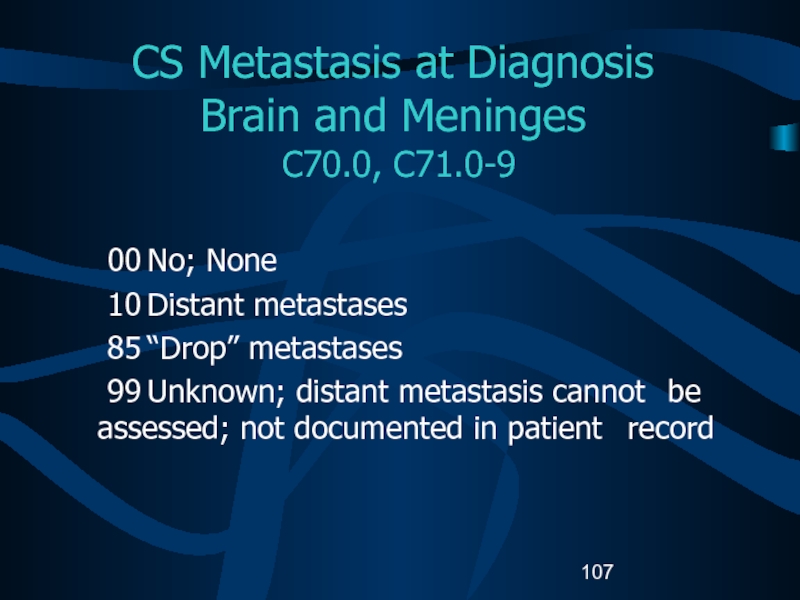
Слайд 107CS Metastasis at Diagnosis
Brain and Meninges
C70.0, C71.0-9
00 No; None
10 Distant metastases
85 “Drop”
metastases
99 Unknown; distant metastasis cannot be assessed; not documented in patient
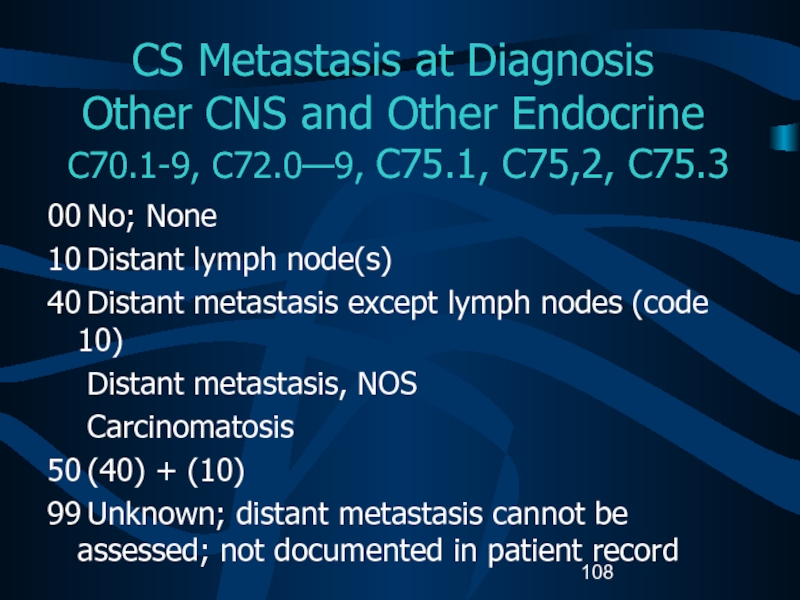
recordСлайд 108CS Metastasis at Diagnosis Other CNS and Other Endocrine C70.1-9,
C72.0—9, C75.1, C75,2, C75.3
00 No; None
10 Distant lymph node(s)
40 Distant metastasis except lymph
nodes (code 10)Distant metastasis, NOS
Carcinomatosis
50 (40) + (10)
99 Unknown; distant metastasis cannot be assessed; not documented in patient record
Слайд 109CS Site-specific Factor 1 (1)
C70.0-C70.9, C71.0-C71.9, C72.0-C72.9
010 WHO Grade I
020 WHO
Grade II
030 WHO Grade III
040 WHO Grade IV
999 Clinically diagnosed; grade unknown;
Not
documented in the medical record;Grade unknown, NOS
Слайд 110CS Site-specific Factor 1 (2)
C70.0-C70.9, C71.0-C71.9, C72.0-C72.9
C75.1- C75.3
Code the WHO
grade for CNS tumors in CS Site-specific factor 1.
Do not
code WHO grade in the sixth digit histology data field.Слайд 111Possible Risk Factors
Genetic predispositions for the development of brain tumors
have been identified.
Population-based studies suggest that no more than 4%
are attributed to heredity.Several environmental factors that may be associated with CNS tumors.
Слайд 112Possible Risk Factors
Epstein-Barr virus in the DNA of primary lymphoma
suggests a viral etiology for CNS tumors.
Reference: “Surveillance of Primary
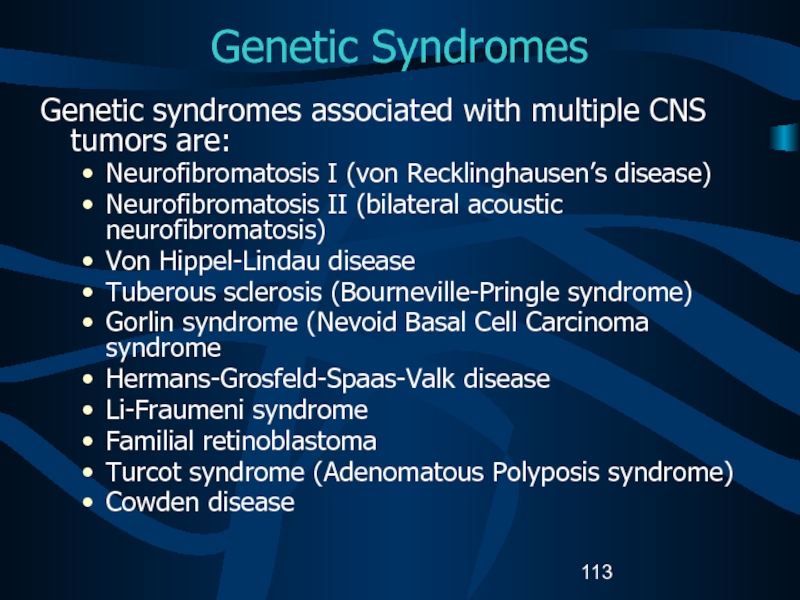
Intracranial and Central Nervous System Tumors: Recommendations from the Brain Tumor Working Group.”Слайд 113Genetic Syndromes
Genetic syndromes associated with multiple CNS tumors are:
Neurofibromatosis I
(von Recklinghausen’s disease)
Neurofibromatosis II (bilateral acoustic neurofibromatosis)
Von Hippel-Lindau disease
Tuberous sclerosis
(Bourneville-Pringle syndrome) Gorlin syndrome (Nevoid Basal Cell Carcinoma syndrome
Hermans-Grosfeld-Spaas-Valk disease
Li-Fraumeni syndrome
Familial retinoblastoma
Turcot syndrome (Adenomatous Polyposis syndrome)
Cowden disease
Слайд 114Diagnostic Tools – Physical Exam
Neurological examination
Eye movements
Vision
Hearing
Reflexes
Balance and coordination
Sense
of smell and touch
Abstract thinking
Memory
Слайд 115Diagnostic Tools: Radiology
Computerized tomography (CT) scan
Magnetic resonance imaging (MRI)
Positron
emission tomography (PET)
Single photon emission computed tomography (SPECT)
Magnetoencephalography (MEG)
Angiography
Слайд 116Diagnostic Tools: Laboratory tests
Audiometry
Electroencephalogram (EEG)
Endocrine evaluation
Evoked potentials
Lumbar puncture
Myelogram
Perimetry
Слайд 117Diagnostic Tools
Needle biopsy
Needle inserted through a burr hole and tissue
extracted for tissue diagnosis.
Stereotactic biopsy
Computer used to guided needle biopsy
to extract tissue. Слайд 118College of American Pathologist
(CAP) Protocols
Site-specific checklists
Required to be
completed in the health record in hospitals with COC-approved cancer
programs for cases diagnosed January 1, 2004 and later.www.cap.org/cancerprotocols/protocols_index.html.
Слайд 119Brain and Spinal Cord
CAP Protocols (1)
Macroscopic
Specimen type
Specimen size
Tumor site
Tumor size
Слайд 120Brain and Spinal Cord
CAP Protocols
Microscopic
Histologic type
Histologic grade
Margins
Additional studies*
Additional pathologic findings*
Comments*
*Not
required for COC approval.
Слайд 121Treatment (1)
Watchful waiting
Surgery
Radiation
Chemotherapy
Hormonal therapy
Immunotherapy
Hematologic Transplant
and Endocrine procedures
Слайд 122Treatment (2)
Inoperable or inaccessible tumors may be treated with primary
radiation and other systemic therapy:
Chemotherapy, immunotherapy, and hormone therapy.
Shunt
insertion to reduce intracranial swelling is not coded as surgical treatment.Слайд 123Surgical Procedure of Primary Site
Brain: Site-specific surgery codes
Meninges
Brain
Spinal cord, cranial
nerves, other CNS.
All Other Sites: Site-specific surgery codes
Pituitary
glandCraniopharyngeal duct
Pineal gland.
Слайд 124Surgical Procedure of Primary Site
C70-0-C70.9, C71.0-C71.9, C72.0-C72.9 (1)
Code 10: Tumor
destruction, NOS
Laser surgery
Laser surgery with photodynamic therapy
Ultrasonic aspirator.
No
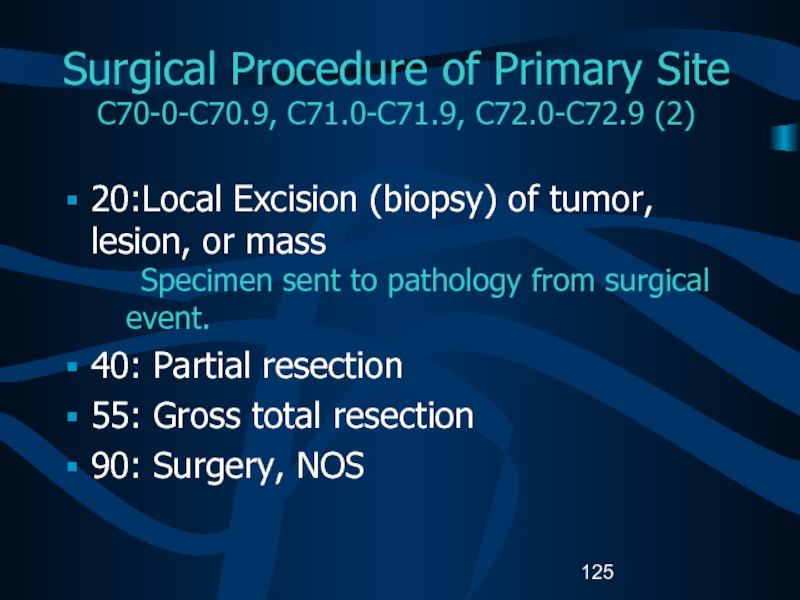
specimen sent to pathology from surgical procedure.Слайд 125Surgical Procedure of Primary Site
C70-0-C70.9, C71.0-C71.9, C72.0-C72.9 (2)
20:Local Excision (biopsy)
of tumor, lesion, or mass
Specimen sent to pathology from surgical
event.40: Partial resection
55: Gross total resection
90: Surgery, NOS
Слайд 126Surgical Procedure of Primary Site
C75.1, C75.2, C75.3 (1)
Code 10: Local
tumor destruction, NOS
Code 11: Photodynamic therapy
Code 12: Electrocautery; fulguration
Code 13:
CryosurgeryCode 14: Laser
No specimen is sent to pathology from surgical events 10-14.
Слайд 127Surgical Procedure of Primary Site
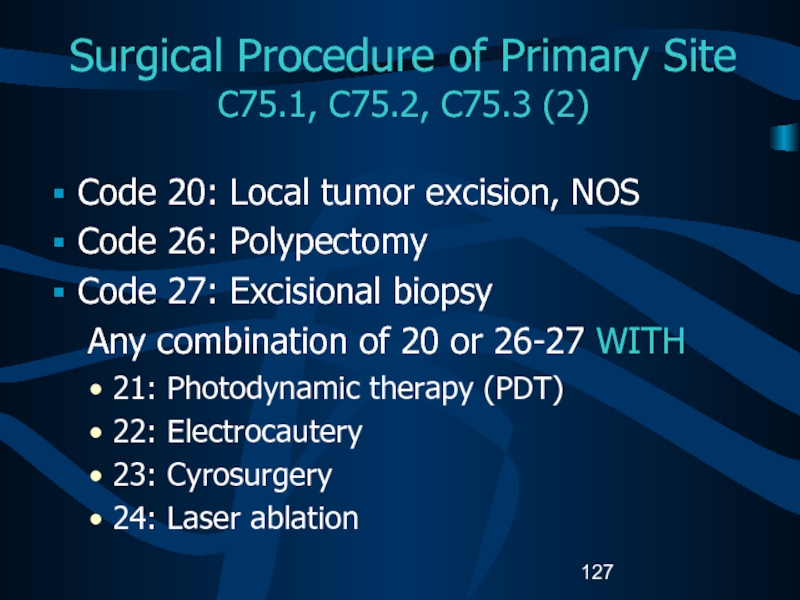
C75.1, C75.2, C75.3 (2)
Code 20: Local
tumor excision, NOS
Code 26: Polypectomy
Code 27: Excisional biopsy
Any combination
of 20 or 26-27 WITH21: Photodynamic therapy (PDT)
22: Electrocautery
23: Cyrosurgery
24: Laser ablation
Слайд 128Surgical Procedure of Primary Site
C75.1, C75.2, C75.3 (3)
Code 25: Laser
excision
Specimen sent to pathology from surgical event 20-27.
Code 30: Simple
or partial surgical removal of primary site. Слайд 129Surgical Procedure of Primary Site
C75.1, C75.2, C75.3 (4)
Code 40: Total
surgical removal of primary site; enucleation
Code 50: Surgery stated to
be “debulking” Code 60: Radical surgery
Partial or total removal of the primary site WITH resection in continuity (partial or total removal) with other organs
Code 90: Surgery, NOS
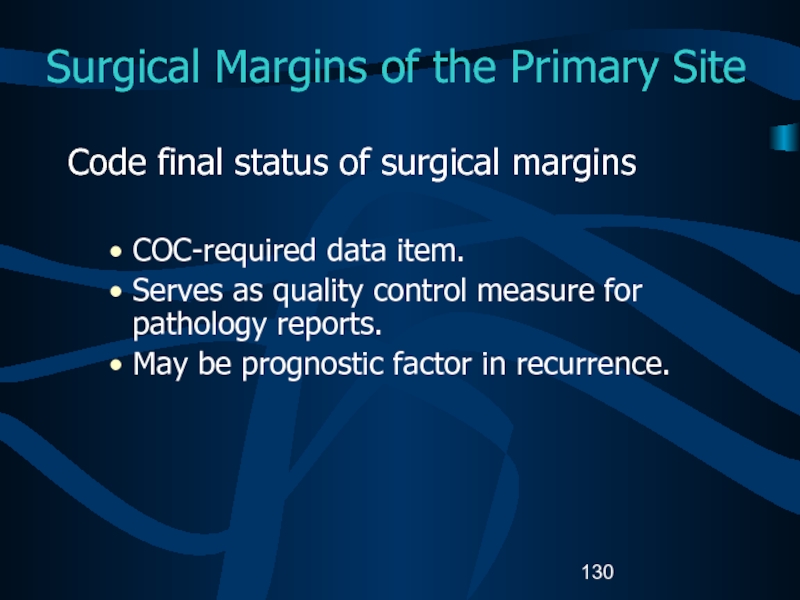
Слайд 130Surgical Margins of the Primary Site
Code final status of surgical
margins
COC-required data item.
Serves as quality control measure for pathology reports.
May be prognostic factor in recurrence.
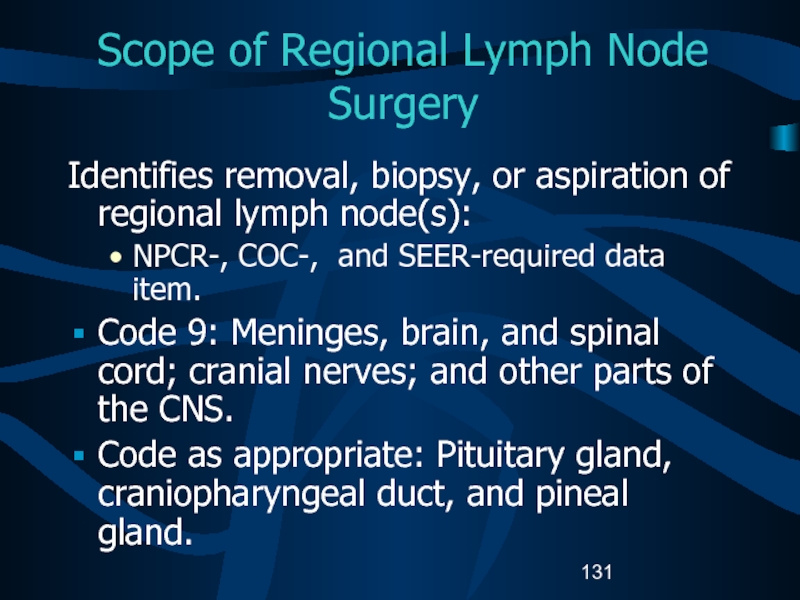
Слайд 131Scope of Regional Lymph Node Surgery
Identifies removal, biopsy, or aspiration
of regional lymph node(s):
NPCR-, COC-, and SEER-required data item.
Code 9:
Meninges, brain, and spinal cord; cranial nerves; and other parts of the CNS.Code as appropriate: Pituitary gland, craniopharyngeal duct, and pineal gland.
Слайд 132Radiation Therapy (1)
Radiation codes indicate type of radiation therapy
performed as part of the first course of treatment.
Records modality
of radiation therapy used to deliver significant regional dose to the primary volume of interest.COC-required data item.
SEER collects these data from COC-approved facilities
NPCR: Supplementary or recommended.
Слайд 133Radiation Therapy (2)
Beam radiation
Codes 20 – 29:
Conventional radiation therapy:
from an external beam directed at the tumor.
Energy is
orthovoltage, cobalt, photon, and/or electron.Code 30: Boron neutron capture therapy (BNCT)
Code 31: Intensity-modulated radiation therapy (IMRT)
Слайд 134Radiation Therapy (3)
Beam radiation
Code 32: Conformal radiation
Code 40: Particle
or proton beam
Code 41: Stereotactic radiosurgery, NOS
Code 42: Linac radiosurgery
Code
43: Gamma knifeСлайд 135Radiation Therapy (3)
Tumors typically treated with stereotactic radiosurgery include:
Acoustic
neuroma
Chordoma
Pineal tumor
Astrocytoma
Craniopharyngioma
Hemangioblastoma
Pituitary adenomal tumor
Слайд 136Radiation Therapy (4)
Radioactive implants
Code 50: Brachytherapy, radiation implants, radiation seeding,
radioactive implants, interstitial implants, intracavitary radiation NOS
Code 51: Intracavitary radiation
with low dose rate applicators (Cesium- 137, Fletcher applicator)Слайд 137Radiation Therapy (5)
Radioactive implants (continued)
Code 52: Intracavitary radiation with high
dose rate applicator
Code 53: Interstitial radiation with low dose rate
sourcesCode 54: Interstitial radiation with high dose rate sources
Code 55: Low dose rate interstitial or intracavitary radium
Слайд 138Chemotherapy (1)
Record type of chemotherapy administered as first course of
treatment:
Code 01: Chemotherapy, NOS
Code 02: Single-agent chemotherapy
Code 03: Multi-agent chemotherapy
Слайд 139Chemotherapy (2)
Blood-brain barrier
Protects the brain from foreign substances, including
chemotherapy.
May be disrupted by receptor-mediated permeabilizers.
Intrathecal chemotherapy
Drugs directly injected into
the cerebrospinal fluid by spinal injection or Ommaya reservoir.Слайд 140Chemotherapy (3)
Interstitial chemotherapy
Administered directly to involved tissues.
Polymer wafers soaked in
a chemotherapeutic agent are inserted in the tumor bed after
tumor resection.Слайд 141Hormone Therapy
Record systemic hormonal agents administered as first course of
treatment.
Tamoxifen and RU-486 (Mifepristone) may be used to treat meningioma.
Steroids
given to treat swelling caused by CNS tumors are not coded as hormone therapy.Слайд 142Immunotherapy (1)
Record whether immunotherapeutic agents were administered as first course
of treatment:
Angiogenesis inhibitors block the development of new blood vessels
and starve the tumor. Interleukins are growth factors that manipulate the tumor’s ability to grow.
Слайд 143Immunotherapy (2)
Gene therapy replaces or repairs the gene responsible for
tumor growth.
Vaccine therapy allows the immune system to detect the
tumor antigens and attack the tumor cells.Слайд 144Hematologic Transplant and Endocrine Procedures
Identify systemic therapeutic procedures administered as
first course of treatment:
Code 10: Bone marrow transplant, NOS
Code 11:
Autologous bone marrow transplantCode 12: Allogeneic bone marrow transplant
Code 20: Stem cell harvest
Code 30: Endocrine surgery and/or endocrine radiation therapy
Code 40: Combination of endocrine surgery and/or radiation with transplant procedure
Слайд 145Technical Issues
Edit Checks
NAACCR Edits Committee is developing and modifying data
edits to accommodate data collection of non-malignant CNS tumors.
Слайд 146Technical Issues
Data Analysis Recommendations
Report and analyze data for non-malignant CNS
tumors separately from malignant tumors.
Footnote that pilocytic astrocytomas are included
in the analysis for malignant brain tumors for continuity of trends.Review the standard site and histology groupings for tabulating estimates of these tumors to allow comparability of information across registries.
Слайд 147References
Manuals, Articles, Reports
A Primer of Brain Tumors, 1998; American Brain
Tumor Association, Des Plaines, IL; 800-886-2282 (can link to the
manual through their website: www.abta.org)Gershman S, Surawicz T, McLaughlin V, Rousseau V. Completeness of Reporting of Brain and Other Central Nervous System Neoplasms. Journal of Registry Management, Winter 2001, Volume 28, Number 4.
Слайд 148References
Manuals, Articles, Reports (continued)
Fritz A, Percy C, Jack V, Shanmugaratnam
K, Sobin V, Parkin D M , Whelan S. International
Classification of Diseases for Oncology, 3rd ed. Geneva: World Health Organization, 2000Report: Surveillance of Primary Intracranial and Central Nervous System Tumors: Recommendations from the Brain Tumor Working Group, National Coordinating Council for Cancer Surveillance, September 1998
Слайд 149References
Websites
American Brain Tumor Association www.abta.org
American College of Surgeons, Commission on
Cancer Information, Facility Oncology Data Standards (FORDS) www.facs.org/dept/cancer/index.html
American Joint Committee
on Cancer, Collaborative Stage Documentation www.edits.cx/cs/Слайд 150References
Websites (continued)
Brain and Neurosurgery Information Center www.brain-surgery.com/index.html
Brain and Spinal Cord
Tumors: Hope through Research www.ninds.nih.gov/health_and_medical/pubs/brain_tumor_hope_through_research.htm
Brain Tumor Guide http://virtualtrials.com/faq/toc.cfm
Central Brain Tumor
Registry of the United States www.cbtrus.org/page2t.htmСлайд 151References
Websites (continued)
College of American Pathologists (CAP), Protocol – Brain ftp://ftp.cap.org/cancerprotocols/Brain03_p.doc
Illustrated
Glossary of Radiology: Anatomy, Examinations and Procedures; Department of Radiology
and Radiological Services, The Uniformed Services University of the Health Scienceshttp://rad.usuhs.mil/glossary.html
Слайд 152References
Websites (continued)
International RadioSurgery Association www.isra.org/index.html
National Brain Tumor Radiosurgery Association www.braintumors.com/radiosurgery/radiosrugery.info#TWO
NCI
Brain Tumor Home Page www.nci.nih.gov/cancer_information/cancer_type/brain_tumor/
Слайд 153References
Websites (continued)
PDQ Cancer Information Summaries: Adult Treatment www.cancer.gov/cancerinfo/pdq/adulttreatment
PDQ Cancer Information
Summaries: Pediatric Treatment www.cancer.gov/cancerinfo/pdq/pediatrictreatment
The Brain Tumor Foundation www.braintumorfoundation.org/neurosurgery/ss3_3.htm
Слайд 154Acknowledgments (1)
Prepared by
Shannon Vann, CTR
for the
North American Association of Central
Cancer Registries (NAACCR)
This training presentation was supported by contract #200-2001-00044
from CDC. The content of this training presentation does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.Слайд 155Acknowledgments (2)
Sponsors
Centers for Disease Control and Prevention
National Program for Cancer
Registries
National Cancer Institute
Surveillance, Epidemiology and End Results Program
North American Association
of Central Cancer RegistriesAmerican Joint Committee on Cancer
Collaborative Stage Task Force
Слайд 156Acknowledgments (3)
CDC National Program of Cancer Registries Planning Committee
Kimberly Cantrell
Gayle
G. Clutter
Faye Floyd
Michael Lanzilotta
Frances Michaud
Слайд 157Acknowledgments (4)
Materials Review Committee
Trista Aarnes-Leong St. Vincent Medical Center, NAACCR Registry
Operations Subcommittee,
Susan Bolick-Aldrich South Carolina Central Cancer Registry, NAACCR Registry Operations
Subcommittee, Chair, Co-chair, Registry Operations Committee Gayle Clutter CDC National Program of Cancer Registries, Registry Operations Subcommittee, National Coordination Council on Cancer Surveillance Brain Tumor Working Group, Chair
Faye Floyd CDC National Program of Cancer Registries
April Fritz NCI Surveillance, Epidemiology and End Results Program, Registry Operations Subcommittee
Elaine Hamlyn Canadian Cancer Registry, Registry Operations Subcommittee,
Holly Howe North American Association of Central Cancer Registries, Executive Director
Betsy Kohler New Jersey State Cancer Registry, NAACCR Education Committee
Carol Kruchko Central Brain Tumor Registry of the United States, Registry Operations Subcommittee, National Coordination Council on Cancer Surveillance Brain Tumor Working Group
Donna Morrel Cancer Surveillance Program of Los Angeles. Registry Operations Subcommittee
Linda Mulvihill North Carolina Central Cancer Registry, Registry Operations Subcommittee
Wendy Scharber Minnesota Cancer Surveillance Program
James Smirniotopoulos Professor of Radiology, Uniformed Services University, Registry Operations Subcommittee
Katheryne Vance California Cancer Registry, Registry Operations Subcommittee
Valerie Vesich American College of Surgeons, Commission on Cancer, Registry Operations Subcommittee