Слайд 1LECTURE 9
Chemistry of Coordination Compounds
30.03.2017
Слайд 2LECTURE OBJECTIVES:
After reading this lesson, the learner will be able
to
state the postulates of Werner’s theory;
define ligands, coordination number
and coordination sphere;
name simple complexes by IUPAC system;
explain valance bond theory;
explain the applications of coordination compounds in extraction of metals, medicine and qualitative analysis.
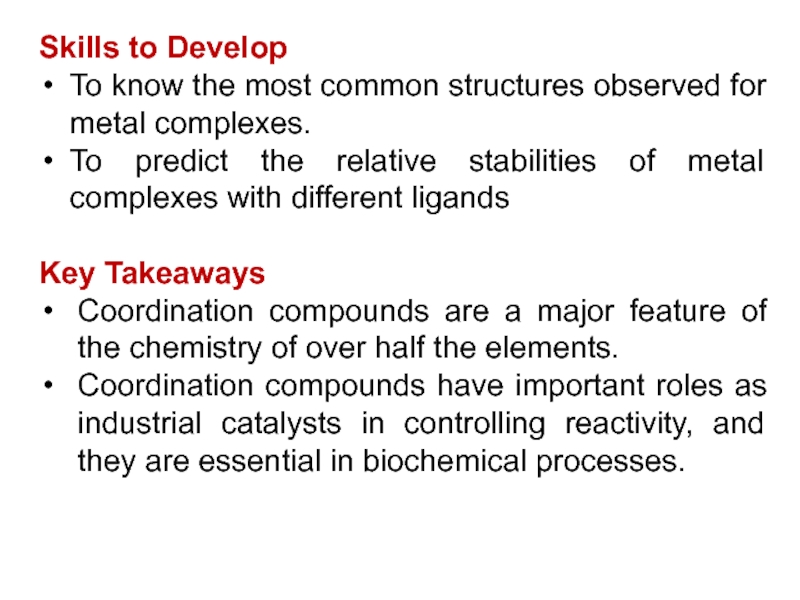
Слайд 3Skills to Develop
To know the most common structures observed for
metal complexes.
To predict the relative stabilities of metal complexes with
different ligands
Key Takeaways
Coordination compounds are a major feature of the chemistry of over half the elements.
Coordination compounds have important roles as industrial catalysts in controlling reactivity, and they are essential in biochemical processes.
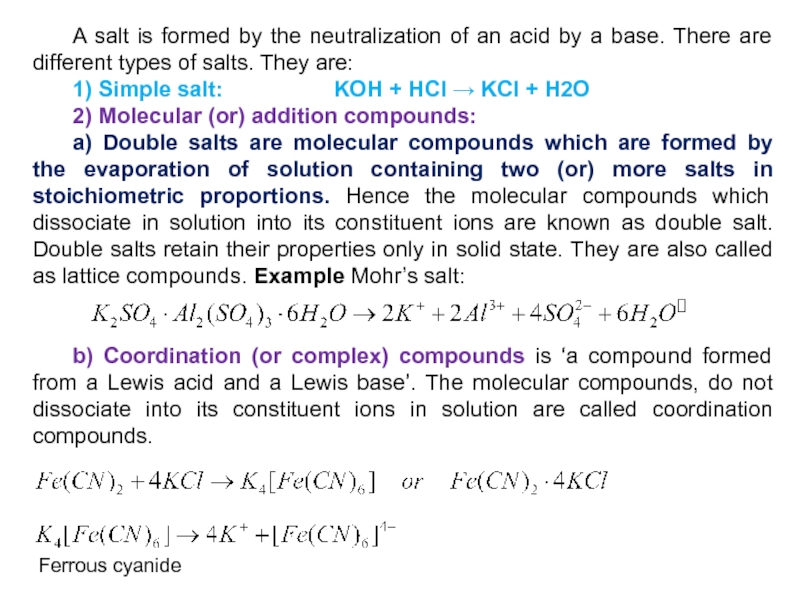
Слайд 4 A salt is formed by the neutralization of an acid
by a base. There are different types of salts. They
are:
1) Simple salt: KOH + HCl → KCl + H2O
2) Molecular (or) addition compounds:
a) Double salts are molecular compounds which are formed by the evaporation of solution containing two (or) more salts in stoichiometric proportions. Hence the molecular compounds which dissociate in solution into its constituent ions are known as double salt. Double salts retain their properties only in solid state. They are also called as lattice compounds. Example Mohr’s salt:
b) Coordination (or complex) compounds is ‘a compound formed from a Lewis acid and a Lewis base’. The molecular compounds, do not dissociate into its constituent ions in solution are called coordination compounds.
Ferrous cyanide
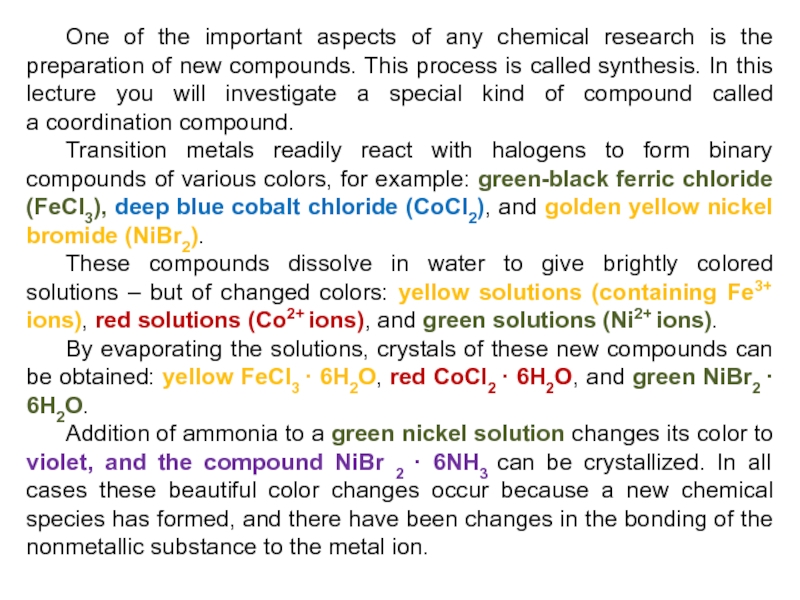
Слайд 5One of the important aspects of any chemical research is
the preparation of new compounds. This process is called synthesis. In
this lecture you will investigate a special kind of compound called a coordination compound.
Transition metals readily react with halogens to form binary compounds of various colors, for example: green-black ferric chloride (FeCl3), deep blue cobalt chloride (CoCl2), and golden yellow nickel bromide (NiBr2).
These compounds dissolve in water to give brightly colored solutions – but of changed colors: yellow solutions (containing Fe3+ ions), red solutions (Co2+ ions), and green solutions (Ni2+ ions).
By evaporating the solutions, crystals of these new compounds can be obtained: yellow FeCl3 · 6H2O, red CoCl2 · 6H2O, and green NiBr2 · 6H2O.
Addition of ammonia to a green nickel solution changes its color to violet, and the compound NiBr 2 · 6NH3 can be crystallized. In all cases these beautiful color changes occur because a new chemical species has formed, and there have been changes in the bonding of the nonmetallic substance to the metal ion.
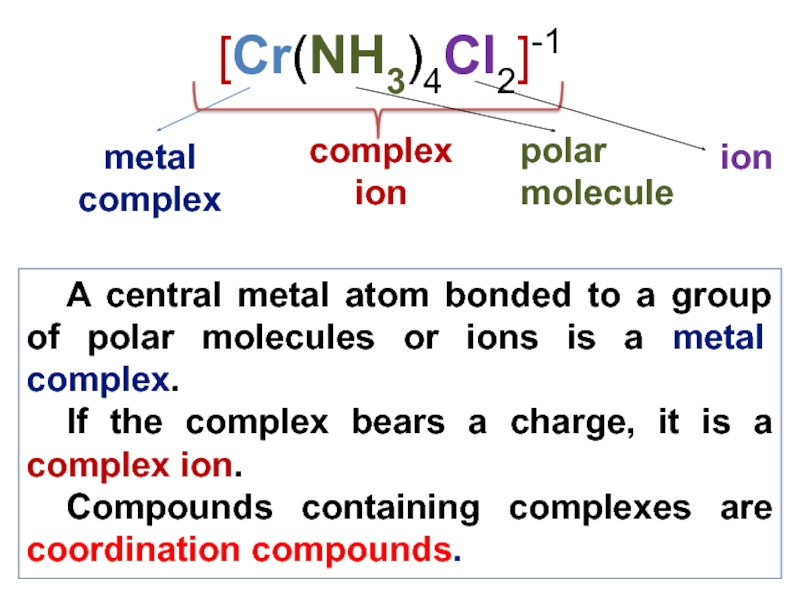
Слайд 7 A central metal atom bonded to a group of polar
molecules or ions is a metal complex.
If the complex bears
a charge, it is a complex ion.
Compounds containing complexes are coordination compounds.
[Cr(NH3)4Cl2]-1
metal complex
complex ion
polar molecule
ion
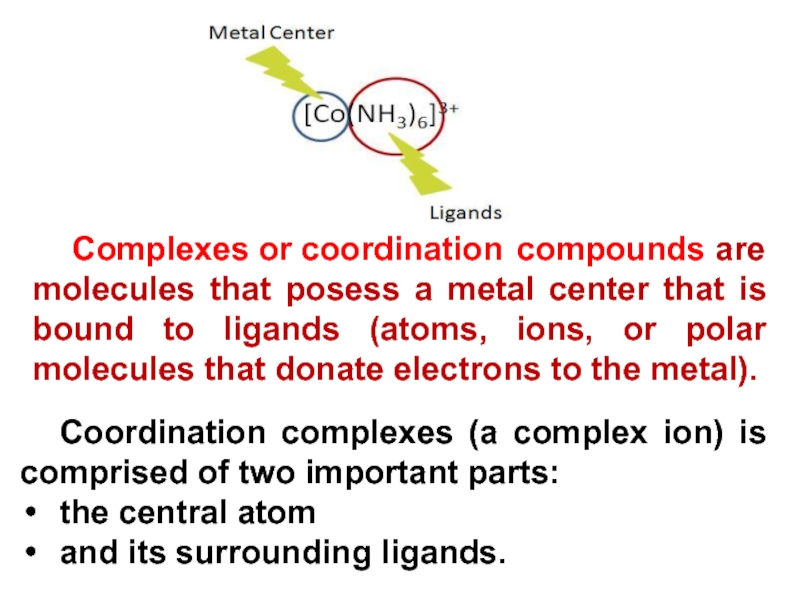
Слайд 8Coordination complexes (a complex ion) is comprised of two important
parts:
the central atom
and its surrounding ligands.
Complexes or coordination compounds are
molecules that posess a metal center that is bound to ligands (atoms, ions, or polar molecules that donate electrons to the metal).
Слайд 9THE CENTRAL ATOM
It is can be:
any metallic ion (usually
a transition d- or f-metal);
nonmetals (B, Si);
p-element (Al).
The overall it
charge can be positive, negative, or neutral.
Thus, in the complex ion an acceptor accepts a pair of electrons from the donor atoms. The acceptor is usually a metal / metal ion to which one (or) more of neutral molecules (or) anions are attached. The acceptor metal cation is referred to as central metal cation. Hence, central metal cation in a complex serves as a lewis acid.
Слайд 10Coordination compounds are complex or contain complex ions, for example:
Complex
Cation: [Co(NH3)6]3+
Complex Anion: [CoCl4(NH3)2]−1
Neutral Complex: [CoCl3(NH3)3]
Coordination Compound: K4[Fe(CN)6]
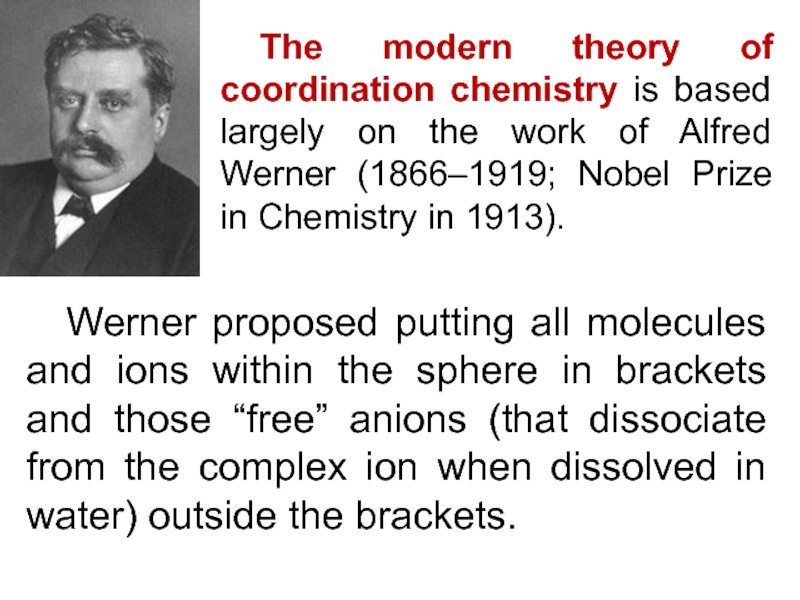
Слайд 11 The modern theory of coordination chemistry is based largely on
the work of Alfred Werner (1866–1919; Nobel Prize in Chemistry
in 1913).
Werner proposed putting all molecules and ions within the sphere in brackets and those “free” anions (that dissociate from the complex ion when dissolved in water) outside the brackets.
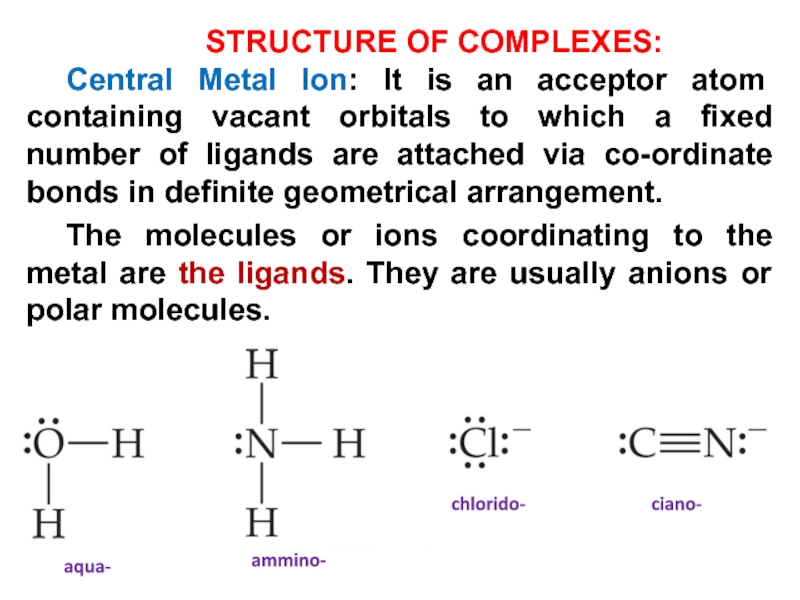
Слайд 12STRUCTURE OF COMPLEXES:
Central Metal Ion: It is an acceptor atom
containing vacant orbitals to which a fixed number of ligands
are attached via co-ordinate bonds in definite geometrical arrangement.
The molecules or ions coordinating to the metal are the ligands. They are usually anions or polar molecules.
aqua-
ammino-
chlorido-
ciano-
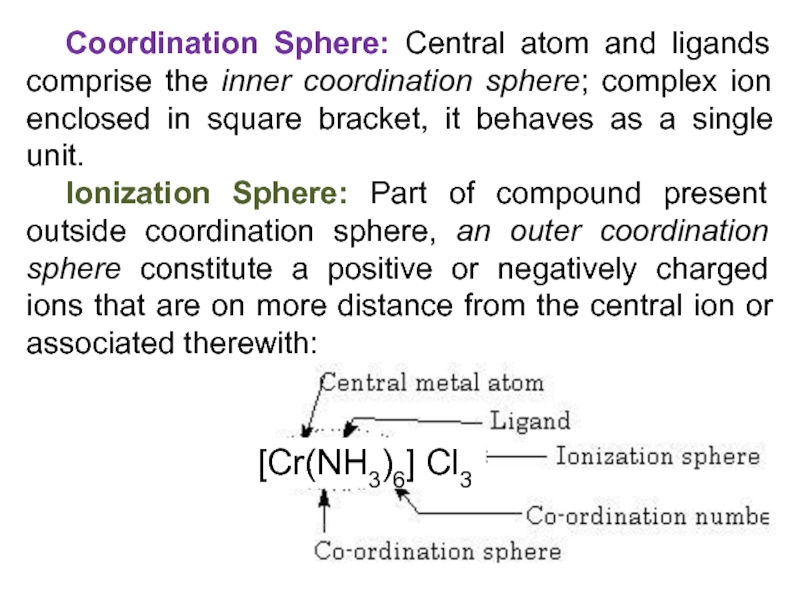
Слайд 13Coordination Sphere: Central atom and ligands comprise the inner coordination
sphere; complex ion enclosed in square bracket, it behaves as
a single unit.
Ionization Sphere: Part of compound present outside coordination sphere, an outer coordination sphere constitute a positive or negatively charged ions that are on more distance from the central ion or associated therewith:
[Cr(NH3)6] Cl3
Слайд 14LIGANDS
It is an ion or polar molecule capable of donating
a pair of electrons to the central atom via a
donor atom.
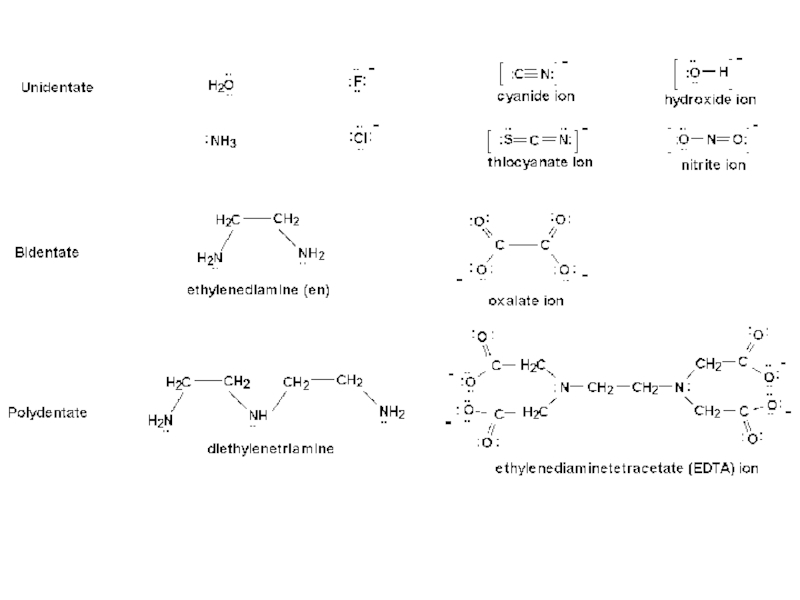
Types of ligands:
Unidentate ligands: Ligands with only one donor atom, e.g. NH3, Cl-, F- etc.
Bidentate ligands: Ligands with two donor atoms, e.g. ethylenediamine, C2O42-(oxalate ion) etc.
Tridentate ligands: Ligands which have three donor atoms per ligand, e.g. (dien) diethyl triamine.
Hexadentate ligands: Ligands which have six donor atoms per ligand, e.g. EDTA.
Chelating Ligands: Multidentate ligand simultaneously co-ordinating to a metal ion through more than one site is called chelating ligand. These ligands produce a ring like structure called chelate. Chelation increases the stability of complex. This effect is called chelation effect.
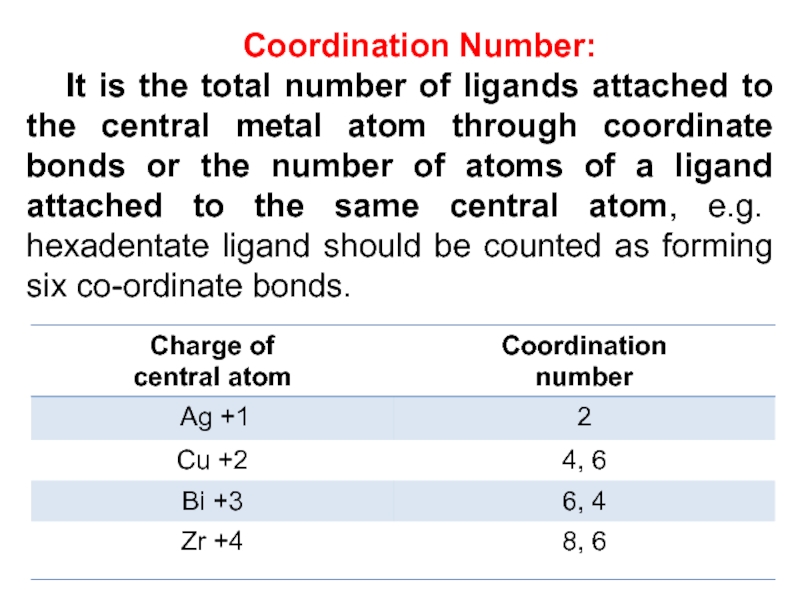
Слайд 16Coordination Number:
It is the total number of ligands attached
to the central metal atom through coordinate bonds or the
number of atoms of a ligand attached to the same central atom, e.g. hexadentate ligand should be counted as forming six co-ordinate bonds.
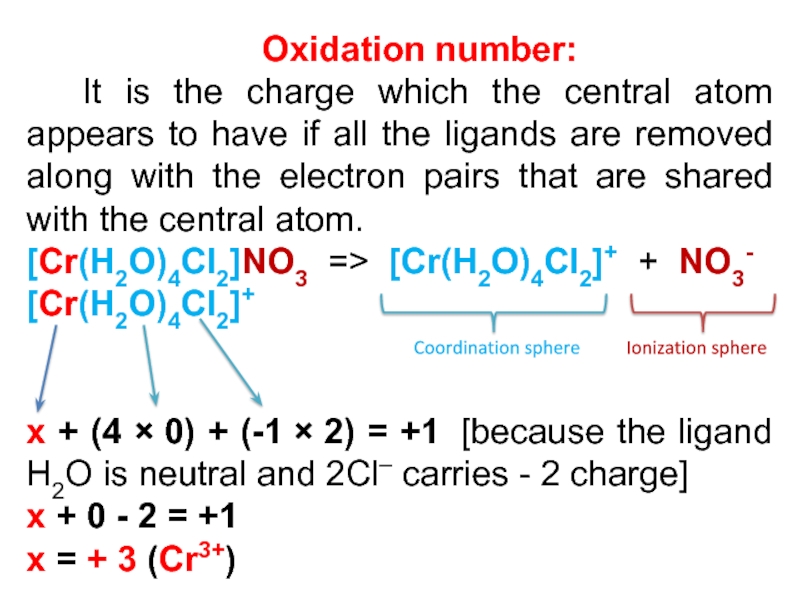
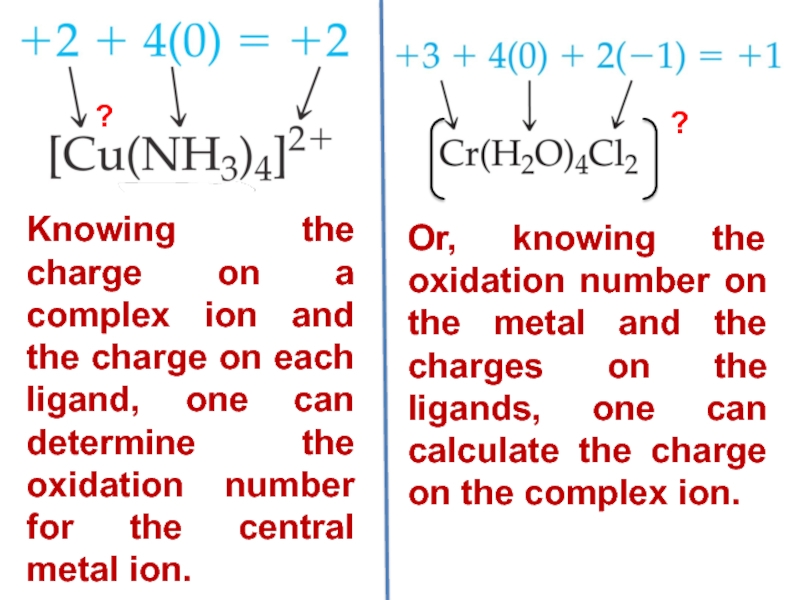
Слайд 17Oxidation number:
It is the charge which the central atom
appears to have if all the ligands are removed along
with the electron pairs that are shared with the central atom.
[Cr(H2O)4Cl2]NO3 => [Cr(H2O)4Cl2]+ + NO3-
[Cr(H2O)4Cl2]+
x + (4 × 0) + (-1 × 2) = +1 [because the ligand H2O is neutral and 2Cl– carries - 2 charge]
x + 0 - 2 = +1
x = + 3 (Cr3+)
Coordination sphere
Ionization sphere
Слайд 18Or, knowing the oxidation number on the metal and the
charges on the ligands, one can calculate the charge on
the complex ion.
Knowing the charge on a complex ion and the charge on each ligand, one can determine the oxidation number for the central metal ion.
?
?
Слайд 19STRUCTURE OF COORDINATION COMPOUND
Cu
Cl
+2
-
[
[
[Cu(NH3)4]Cl2
КОМПЛЕКСООБРАЗОВАТЕЛЬ
LIGANDS
COORDINATION NUMBER- 4
COORDINATION SPHERE
IONIZATION SPHERE
COMPLEX ION
2
NH
3
NH
3
NH
3
+2
Слайд 20 Classification of complex compounds:
By the charge of the complex
ion
[Cr(H2O)4]3+Cl3
[PtCl4(NH3)2] 0
K2[PtCl6]2-
[Cu(NH3)4]2+[PtCl4]2-
Слайд 21By composition of ionization sphere
H2[PtCl6]
Na3[AlF6]
[Pt(NH3)2Cl2]
[Ag(NH3)2]OH
Слайд 22By the nature of the ligand:
[Fe(H2O)6]SO4
K[Au(CN)4]
[Zn(NH3)4]Cl2
[CoCl(NH3)3(H2O)2](NO3)2
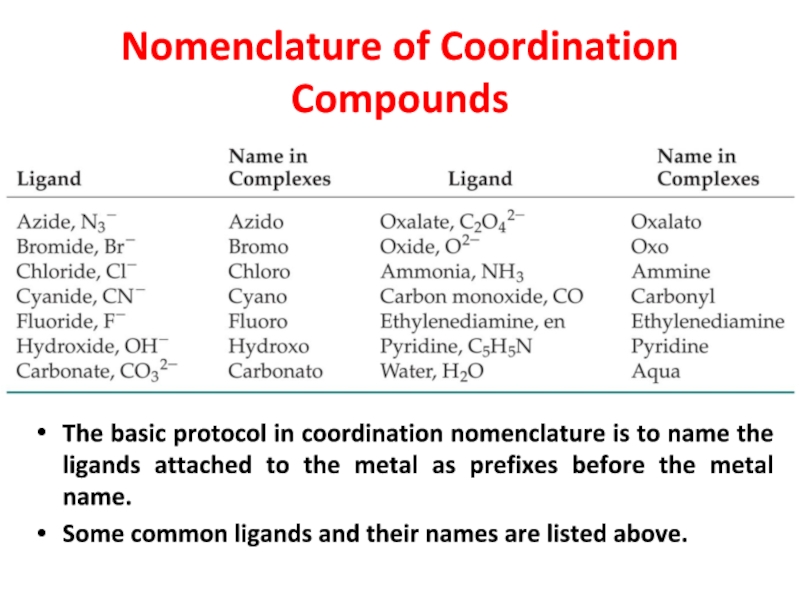
Слайд 23Nomenclature of Coordination Compounds
The basic protocol in coordination nomenclature is
to name the ligands attached to the metal as prefixes
before the metal name.
Some common ligands and their names are listed above.
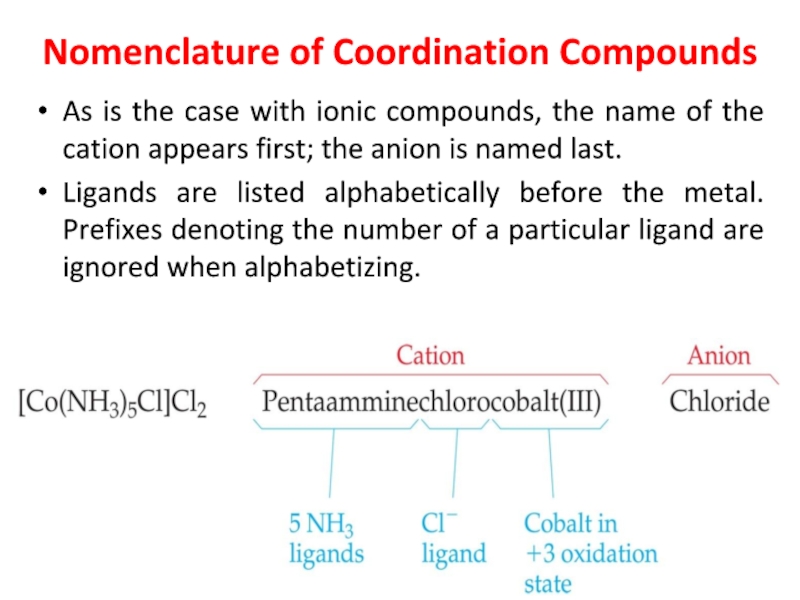
Слайд 27Nomenclature of Coordination Compounds
As is the case with ionic compounds,
the name of the cation appears first; the anion is
named last.
Ligands are listed alphabetically before the metal. Prefixes denoting the number of a particular ligand are ignored when alphabetizing.
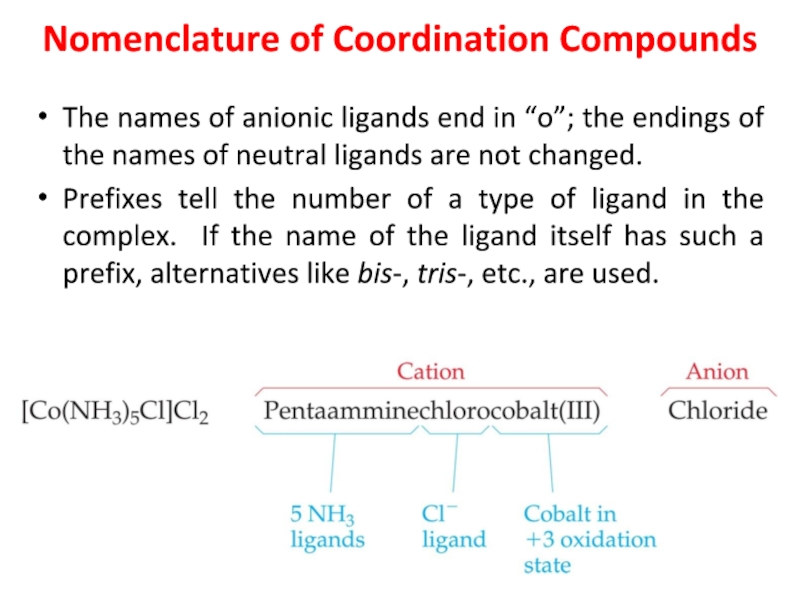
Слайд 28Nomenclature of Coordination Compounds
The names of anionic ligands end in
“o”; the endings of the names of neutral ligands are
not changed.
Prefixes tell the number of a type of ligand in the complex. If the name of the ligand itself has such a prefix, alternatives like bis-, tris-, etc., are used.
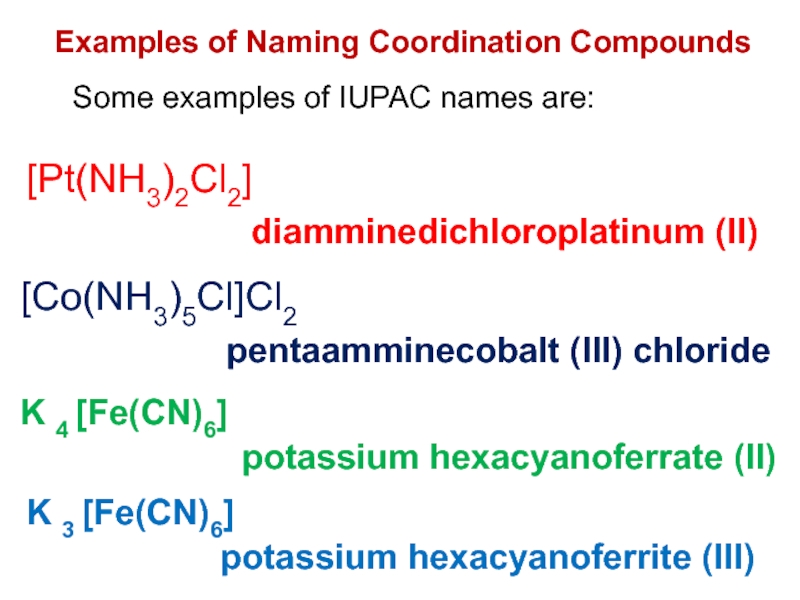
Слайд 29Examples of Naming Coordination Compounds
Some examples of IUPAC names are:
diamminedichloroplatinum
(II)
pentaamminecobalt (III) chloride
potassium hexacyanoferrate (II)
potassium hexacyanoferrite (III)
[Pt(NH3)2Cl2]
[Co(NH3)5Cl]Cl2
K
4 [Fe(CN)6]
K 3 [Fe(CN)6]
Слайд 30Preparation of complex compounds
Complex compounds prepared by such reactions:
1)
Connection reactions:
HgI2 + 2KI → K2[HgI4]
2) Substitution reactions:
[Cu(Н2О)4]SO4 + 4NH3 → [Cu(NH3)4]SO4 + 4Н2О
3) Exchange reactions:
2 ZnCl2 + K4 [Fe(CN)6] → Zn2[Fe(CN)6] + 4KCl
4) Redox reactions:
2Al + 6KOH + 6H2O → K3[Al(OH)6] + 3H2↑
Слайд 31Nature of chemical bonding in complex compounds
Structure physico-chemical and biological
properties of complex compounds depend on the nature of chemical
bonds in them. Currently, the nature of chemical bonds in complex compounds such theories explain:
1) the method of valence bonds ;
2) the crystal field theory ;
3) the method of molecular orbitals .
A coordination compound consists of a central metal ion which is chemically bonded to one or more atoms or groups of atoms by coordinate covalent bonds. The metal ion contains one or more empty orbitals which can receive pair(s) of electrons and the atom or group of atoms bonded to the metal ion (ligands) contain one or more pairs of electrons which can be donated to the metal ion. When a covalent bond (a bond formed by sharing of one or more pairs of electrons) contains a pair of electrons which comes from only one atom in the bond it is called a coordinate covalent bond.
Слайд 32PROPERTIES OF COMPLEX COMPOUNDS
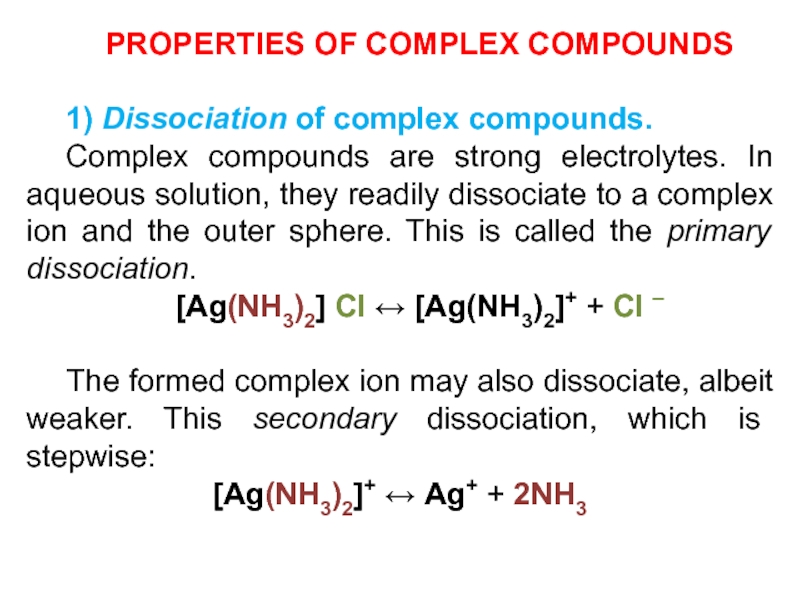
1) Dissociation of complex compounds.
Complex compounds are
strong electrolytes. In aqueous solution, they readily dissociate to a
complex ion and the outer sphere. This is called the primary dissociation.
[Ag(NH3)2] Cl ↔ [Ag(NH3)2]+ + Cl –
The formed complex ion may also dissociate, albeit weaker. This secondary dissociation, which is stepwise:
[Ag(NH3)2]+ ↔ Ag+ + 2NH3
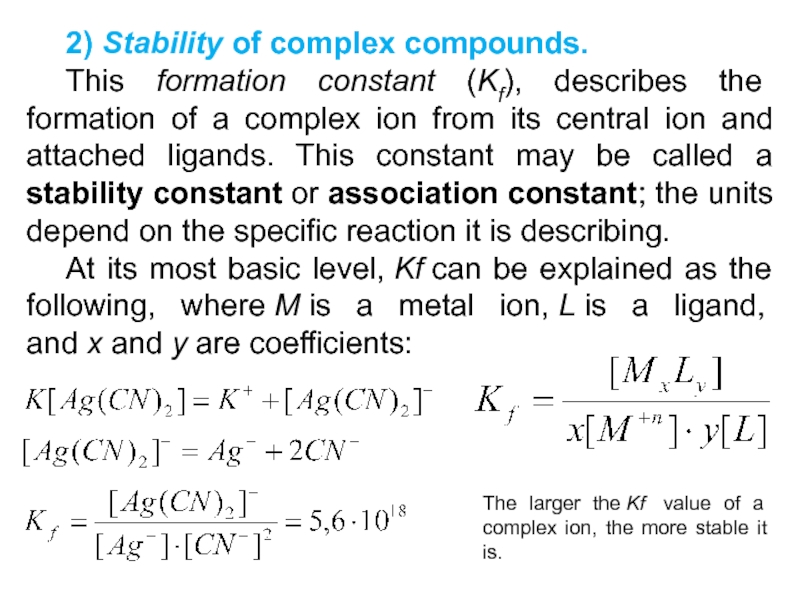
Слайд 332) Stability of complex compounds.
This formation constant (Kf), describes
the formation of a complex ion from its central ion
and attached ligands. This constant may be called a stability constant or association constant; the units depend on the specific reaction it is describing.
At its most basic level, Kf can be explained as the following, where M is a metal ion, L is a ligand, and x and y are coefficients:
The larger the Kf value of a complex ion, the more stable it is.
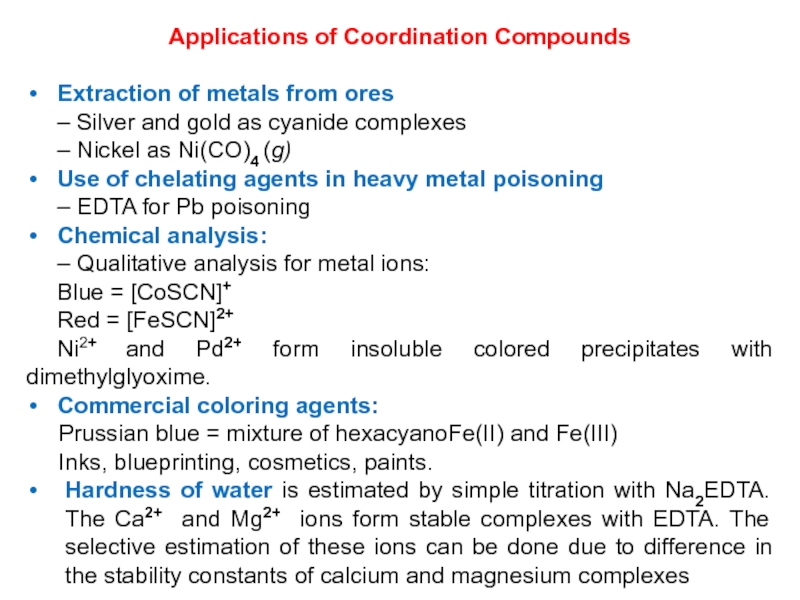
Слайд 34Applications of Coordination Compounds
Extraction of metals from ores
– Silver and
gold as cyanide complexes
– Nickel as Ni(CO)4 (g)
Use of chelating
agents in heavy metal poisoning
– EDTA for Pb poisoning
Chemical analysis:
– Qualitative analysis for metal ions:
Blue = [CoSCN]+
Red = [FeSCN]2+
Ni2+ and Pd2+ form insoluble colored precipitates with dimethylglyoxime.
Commercial coloring agents:
Prussian blue = mixture of hexacyanoFe(II) and Fe(III)
Inks, blueprinting, cosmetics, paints.
Hardness of water is estimated by simple titration with Na2EDTA. The Ca2+ and Mg2+ ions form stable complexes with EDTA. The selective estimation of these ions can be done due to difference in the stability constants of calcium and magnesium complexes



![LECTURE 9 Coordination compounds are complex or contain complex ions, for example: Complex Cation: [Co(NH3)6]3+Complex Anion: [CoCl4(NH3)2]−1Neutral Complex: [CoCl3(NH3)3]Coordination Compound: K4[Fe(CN)6] Coordination compounds are complex or contain complex ions, for example: Complex Cation: [Co(NH3)6]3+Complex Anion: [CoCl4(NH3)2]−1Neutral Complex: [CoCl3(NH3)3]Coordination Compound: K4[Fe(CN)6]](/img/thumbs/9d0a00922733bcdf1b81120709349320-800x.jpg)

![LECTURE 9 STRUCTURE OF COORDINATION COMPOUNDCuCl+2-[[[Cu(NH3)4]Cl2КОМПЛЕКСООБРАЗОВАТЕЛЬLIGANDSCOORDINATION NUMBER- 4COORDINATION SPHEREIONIZATION SPHERECOMPLEX ION2NH3NH3NH3+2 STRUCTURE OF COORDINATION COMPOUNDCuCl+2-[[[Cu(NH3)4]Cl2КОМПЛЕКСООБРАЗОВАТЕЛЬLIGANDSCOORDINATION NUMBER- 4COORDINATION SPHEREIONIZATION SPHERECOMPLEX ION2NH3NH3NH3+2](/img/thumbs/ba516294186ebb74e0215ce4c26d5087-800x.jpg)
![LECTURE 9 Classification of complex compounds: By the charge of the complex ion[Cr(H2O)4]3+Cl3[PtCl4(NH3)2] 0K2[PtCl6]2-[Cu(NH3)4]2+[PtCl4]2- Classification of complex compounds: By the charge of the complex ion[Cr(H2O)4]3+Cl3[PtCl4(NH3)2] 0K2[PtCl6]2-[Cu(NH3)4]2+[PtCl4]2-](/img/thumbs/20f5c96b5605da6eb4b338d8b43639db-800x.jpg)
![LECTURE 9 By composition of ionization sphereH2[PtCl6]Na3[AlF6][Pt(NH3)2Cl2][Ag(NH3)2]OH By composition of ionization sphereH2[PtCl6]Na3[AlF6][Pt(NH3)2Cl2][Ag(NH3)2]OH](/img/thumbs/d4a88dd23491683936f6214c17598667-800x.jpg)
![LECTURE 9 By the nature of the ligand:[Fe(H2O)6]SO4K[Au(CN)4][Zn(NH3)4]Cl2[CoCl(NH3)3(H2O)2](NO3)2 By the nature of the ligand:[Fe(H2O)6]SO4K[Au(CN)4][Zn(NH3)4]Cl2[CoCl(NH3)3(H2O)2](NO3)2](/img/tmb/4/326028/01f162b0a108147be2691727541b2770-800x.jpg)

![LECTURE 9 Preparation of complex compoundsComplex compounds prepared by such reactions: 1) Connection Preparation of complex compoundsComplex compounds prepared by such reactions: 1) Connection reactions: HgI2 + 2KI → K2[HgI4]](/img/thumbs/9f2377b5c2f1e1c058d0cc87f8d70ec6-800x.jpg)