Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Tip: Some icon can able to use ctrl+mouse click to go to datasheet Batteries
Содержание
- 1. Tip: Some icon can able to use ctrl+mouse click to go to datasheet Batteries
- 2. You can download this presentationModify ItFree to DownloadEasy to AccessAsk if questions
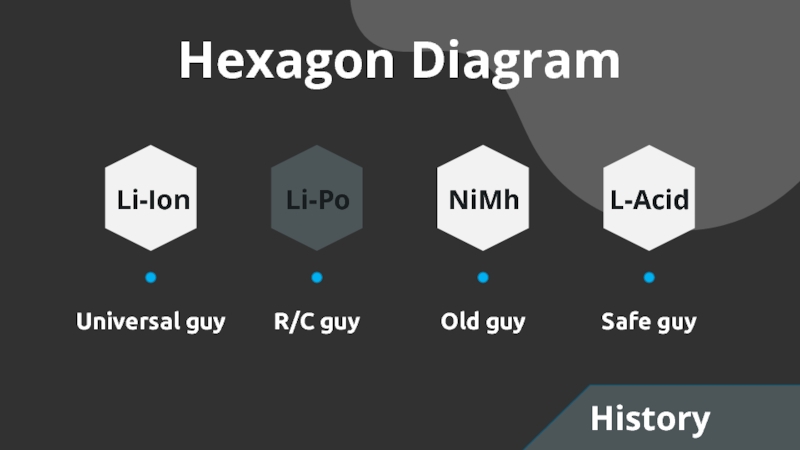
- 3. Hexagon DiagramUniversal guyR/C guyOld guySafe guyLi-IonLi-PoNiMhL-AcidHistory
- 4. A wonderful serenity has taken possession of
- 5. First practical batteriesThe Daniell cell was also
- 6. Rechargeable batteriesA wonderful serenity has taken possession
- 7. first alkaline batteryThe first models were robust
- 8. Lithium and lithium-ion batteriesLithium is the metal with
- 9. Paul EdgusoSony commercialized the lithium-ion battery in 1991. In 1981,
- 10. They generally have a lower energy density than normal
- 11. Li-ionCheap with high energy densityNormalCan hold more current but more expensiveHi-CurrentCheckCheck
- 12. Li-ionHigh capacity per Weight01High capacity per Volume02Cheap03Low
- 13. Li-ionLi-ion batteries provide lightweight, high energy density
- 14. Li-ion Hi-CurrentHigh peak current01High capacity per Weight02High
- 15. Li-ion Hi-CurrentLithium-ion batteries are commonly used for
- 16. Li-poVery High peak current01High capacity per Weight02High work temperature03Over charge and discharge VoltageUnsafety0102
- 17. Li-po
- 18. SupercapacitorOver High peak current01High work temperature (-45 ~ 65℃)02High work life (over 10 years)03Expensive05Low capacitySafety010204Fast charging
- 19. Supercapacitors are used in applications requiring many
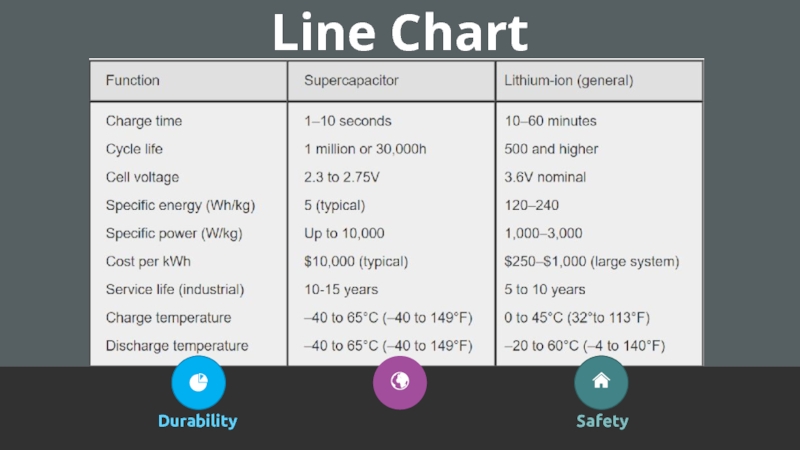
- 20. Line ChartDurabilitySafety
- 21. SECTION BREAKLet’s make a cup of coffee
- 22. The Future of Battery TechnologyThinSaveStep 1Far far
- 23. LCBs (Lithium Ceramic Batteries) High work temperature (-45 ~ 65℃)01Safety02Can flex and bend03Over ExpensiveLow capacity0102
- 24. LCBsThey are extremely flexible and are designed
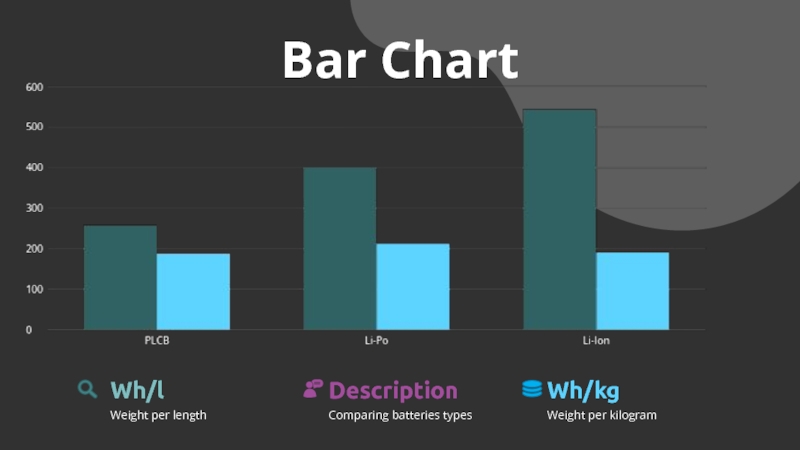
- 25. Bar ChartComparing batteries typesDescriptionWeight per lengthWh/lWeight per kilogramWh/kg
- 26. Слайд 26
- 27. Locationthepowerofdarknes2000@email.comEmailhttps://www.youtube.com/watch?v=LqgP16JQ24IMore sourceJOIN USHope you enjoy this presentationDescriptionhttps://vk.com/test.testirovatAny questions?
- 28. That’s all for now!THANK
- 29. Скачать презентанцию
Слайды и текст этой презентации
Слайд 4A wonderful serenity has taken possession of my entire soul,
like these sweet mornings
In 1780, Luigi Galvani was dissecting a frog affixed
to a brass hook. When he touched its leg with his iron scalpel, the leg twitched. Galvani believed the energy that drove this contraction came from the leg itself, and called it "animal electricity".However, Alessandro Volta, a friend and fellow scientist, disagreed, believing this phenomenon was caused by two different metals joined together by a moist intermediary. In 1800, Volta invented the first true battery, which came to be known as the voltaic pile. The voltaic pile consisted of pairs of copper and zinc discs piled on top of each other, separated by a layer of cloth or cardboard soaked in brine (i.e., the electrolyte).
Слайд 5First practical batteries
The Daniell cell was also used as a
working standard for definition of the volt, which is the unit
of electromotive force.A British chemist named John Frederic Daniell found a way to solve the hydrogen bubble problem in the Voltaic Pile by using a second electrolyte to consume the hydrogen produced by the first. In 1836 he invented the Daniell cell.
The Daniell cell was a great improvement over the existing technology used in the early days of battery development and was the first practical source of electricity. It provided a longer and more reliable current than the Voltaic cell.
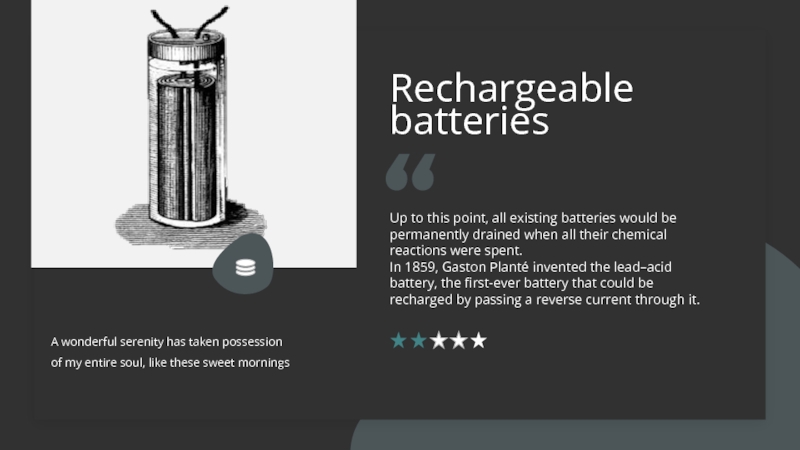
Слайд 6Rechargeable batteries
A wonderful serenity has taken possession of my entire
soul, like these sweet mornings
Up to this point, all existing
batteries would be permanently drained when all their chemical reactions were spent. In 1859, Gaston Planté invented the lead–acid battery, the first-ever battery that could be recharged by passing a reverse current through it.
Слайд 7first alkaline battery
The first models were robust and had significantly
better energy density than lead-acid batteries, but were much more
expensive.NiCd, the first alkaline battery. In 1899, a Swedish scientist named Waldemar Jungner invented the nickel–cadmium battery, the first battery to use an alkaline electrolyte. It was commercialized in Sweden in 1910 and reached the United States in 1946.
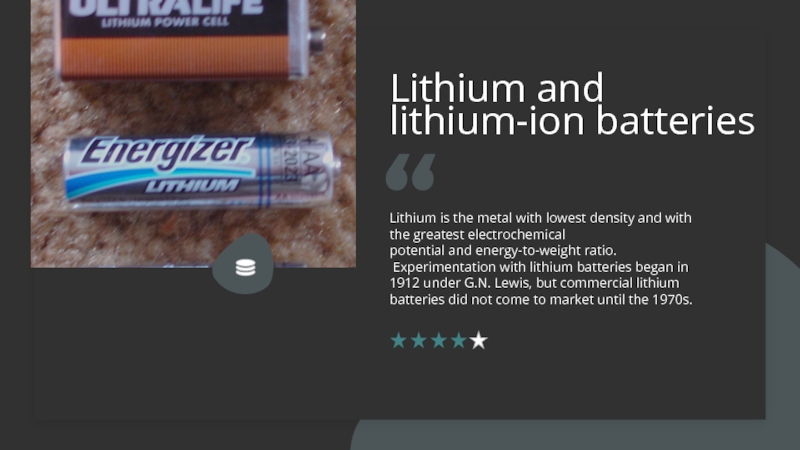
Слайд 8Lithium and lithium-ion batteries
Lithium is the metal with lowest density and
with the greatest electrochemical potential and energy-to-weight ratio.
Experimentation with lithium batteries began in 1912 under G.N.
Lewis, but commercial lithium batteries did not come to market until the 1970s.Слайд 9Paul Edguso
Sony commercialized the lithium-ion battery in 1991.
In 1981, Japanese chemists Tokio Yamabe and Shizukuni
Yata discovered a novel nano-carbonacious-PAS (polyacene,) and found that it was very
effective for the anode in the conventional liquid electrolyte.This led a research team managed by Akira Yoshino of Asahi Chemical, Japan, to build the first lithium-ion battery prototype in 1985, a rechargeable and more stable version of the lithium battery;
Слайд 10They generally have a lower energy density than normal lithium-ion batteries.
In 1997,
the lithium polymer battery was released by Sony and Asahi Kasei.
These
batteries hold their electrolyte in a solid polymer composite instead of in a liquid solvent, and the electrodes and separators are laminated to each other. Слайд 11Li-ion
Cheap with high energy density
Normal
Can hold more current but more
expensive
Hi-Current
Check
Check
Слайд 12Li-ion
High capacity per Weight
01
High capacity per Volume
02
Cheap
03
Low work temperature (~20
℃)
04
Low peak current
Over charge and discharge Voltage
01
02
03
Unsafety
Слайд 13Li-ion
Li-ion batteries provide lightweight, high energy density power sources for
a Electric vehicles
Vehicles
Hing density energy per weigh – ideal for
energy storagePower banks
Slim and compact.
This battery the best solution for portable devices
Portables devices
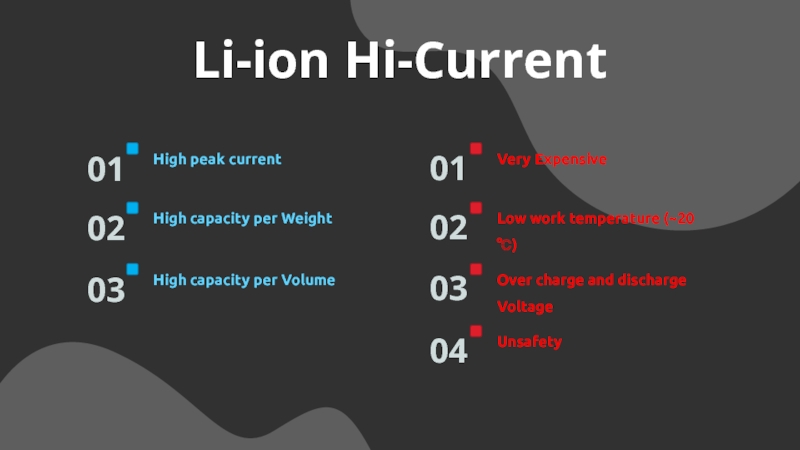
Слайд 14Li-ion Hi-Current
High peak current
01
High capacity per Weight
02
High capacity per Volume
03
Very
Expensive
04
Low work temperature (~20 ℃)
Over charge and discharge Voltage
01
02
03
Unsafety
Слайд 15Li-ion Hi-Current
Lithium-ion batteries are commonly used for portable electronics and
electric vehicles and are growing in popularity for military and
aerospace applications.Description
Research areas for lithium-ion batteries include life extension, energy density, safety, cost reduction, and charging speed, among others.
Description
18560
dimension
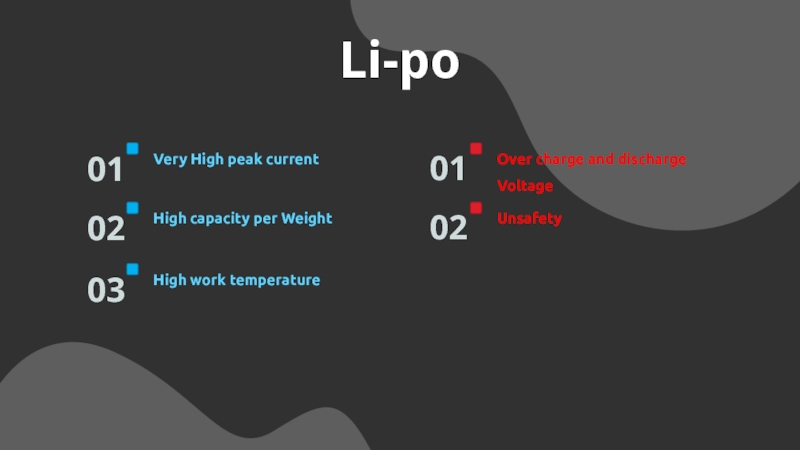
Слайд 16Li-po
Very High peak current
01
High capacity per Weight
02
High work temperature
03
Over charge
and discharge Voltage
Unsafety
01
02
Слайд 18Supercapacitor
Over High peak current
01
High work temperature (-45 ~ 65℃)
02
High work
life (over 10 years)
03
Expensive
05
Low capacity
Safety
01
02
04
Fast charging
Слайд 19Supercapacitors are used in applications requiring many rapid charge/discharge cycles,
rather than long term compact energy storage — in automobiles,
buses, trains, cranes and elevatorsA supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor with a capacitance value much higher than other capacitors, but with lower voltage limits, that bridges the gap between electrolytic capacitors and rechargeable batteries.
More
Supercapacitor
Слайд 22The Future of Battery Technology
Thin
Save
Step 1
Far far away, behind the
word mountains, far from the countries Vokalia and Consonantia, there
live the blind texts.Step 2
Far far away, behind the word mountains, far from the countries Vokalia and Consonantia, there live the blind texts.
Step 3
Far far away, behind the word mountains, far from the countries Vokalia and Consonantia, there live the blind texts.
Step 4
Far far away, behind the word mountains, far from the countries Vokalia and Consonantia, there live the blind texts.
Progress
Far far away, behind the word mountains, far from the countries Vokalia and Consonantia, there live the blind texts.
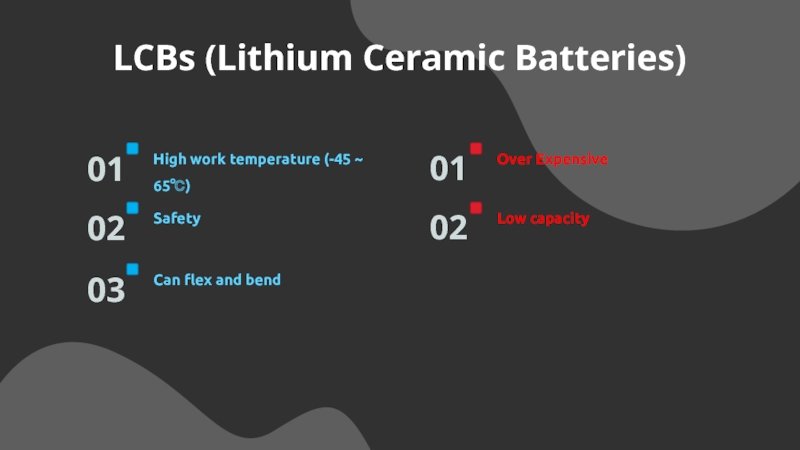
Слайд 23LCBs (Lithium Ceramic Batteries)
High work temperature (-45 ~ 65℃)
01
Safety
02
Can
flex and bend
03
Over Expensive
Low capacity
01
02
Слайд 24LCBs
They are extremely flexible and are designed to withstand a
lot of abuse, including cuts and punctures
“Super flexible and extremely
resilient to damage”Capacity: 105mAh
Nominal Voltage: 3.75V
Dimensions: 50x100mm
Thickness: 0.375mm
0.375mm
these lithium-ceramic batteries are awesome for prototyping wearables and other other ultra-thin devices.
thick
More