Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Atomic physics
Содержание
- 1. Atomic physics
- 2. Atomic Spectra of GasesAll objects emit thermal
- 3. Atomic Spectra of GasesAnother form of spectroscopy
- 4. Atomic Spectra of GasesIn 1885, a Swiss
- 5. Atomic Spectra of GasesOther lines in the
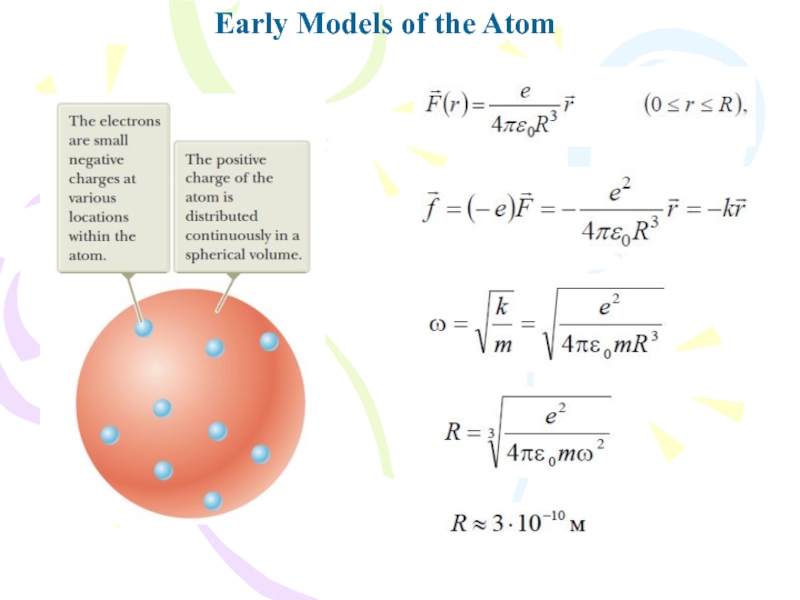
- 6. Early Models of the Atom
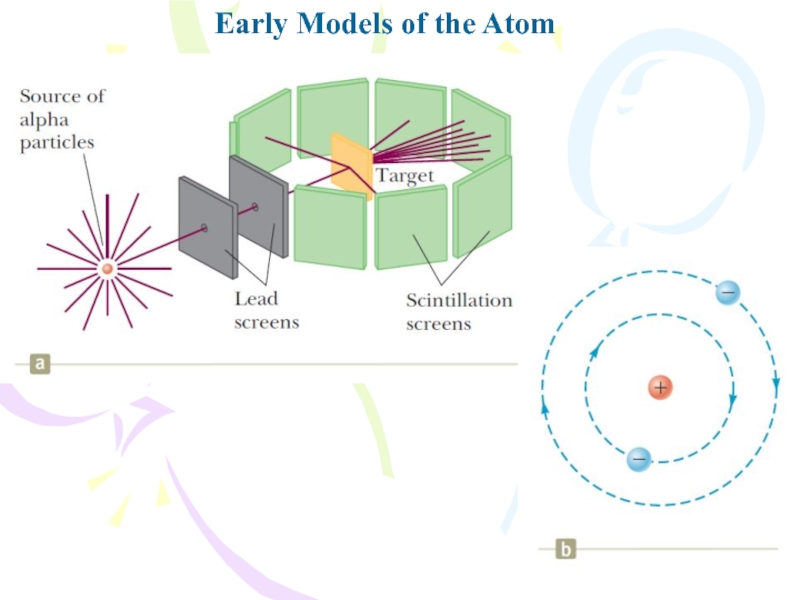
- 7. Early Models of the Atom
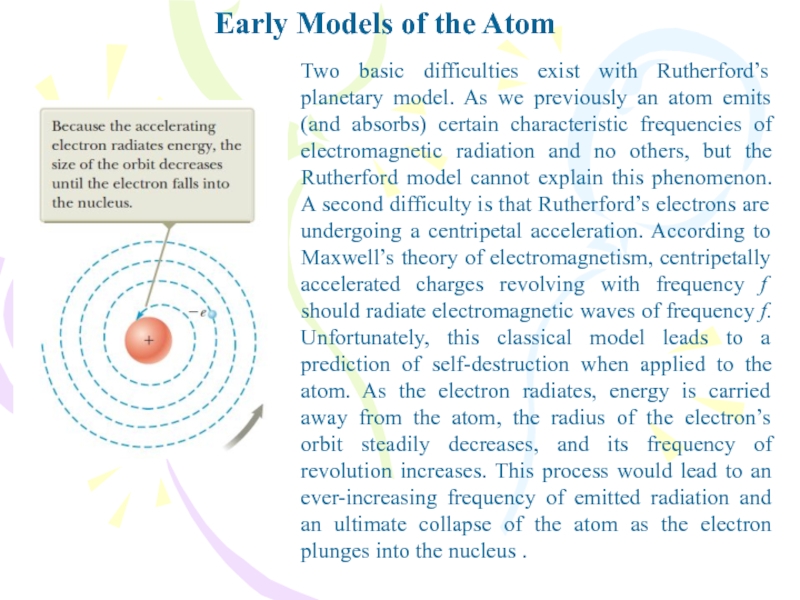
- 8. Early Models of the AtomTwo basic difficulties
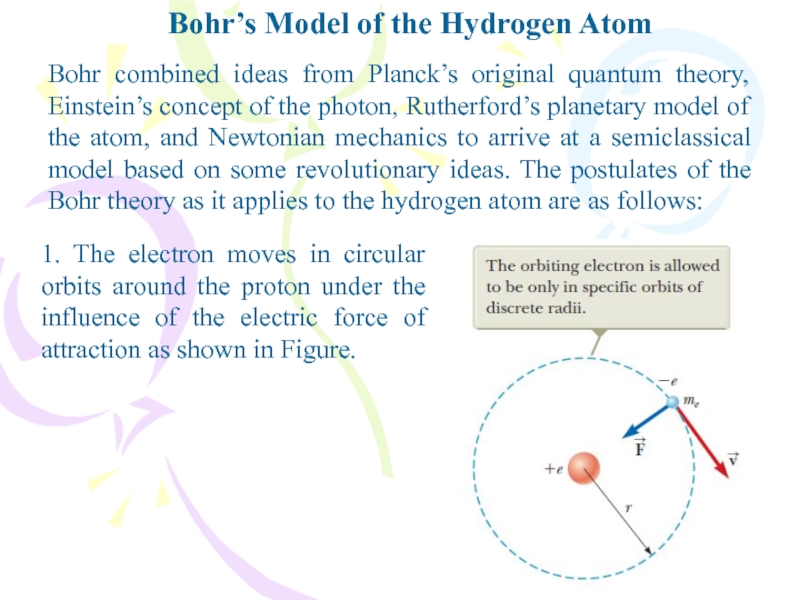
- 9. Bohr’s Model of the Hydrogen AtomBohr combined
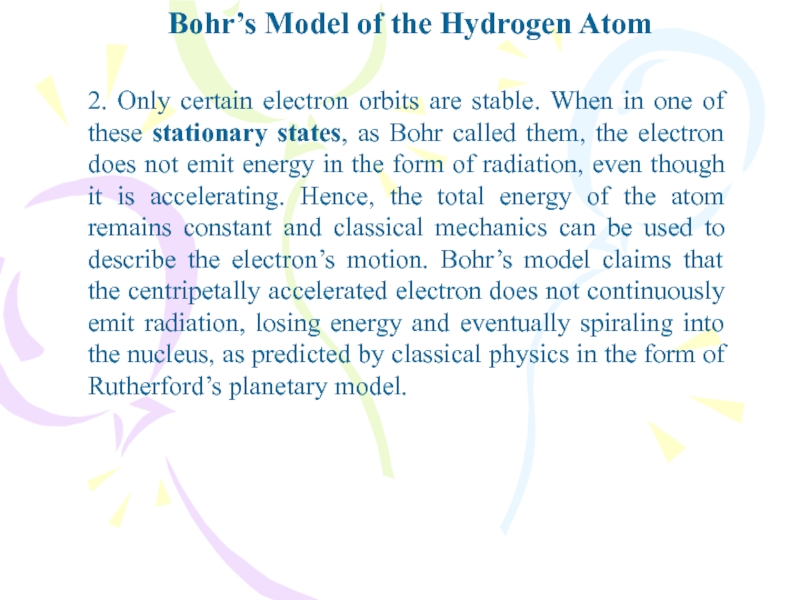
- 10. Bohr’s Model of the Hydrogen Atom2. Only
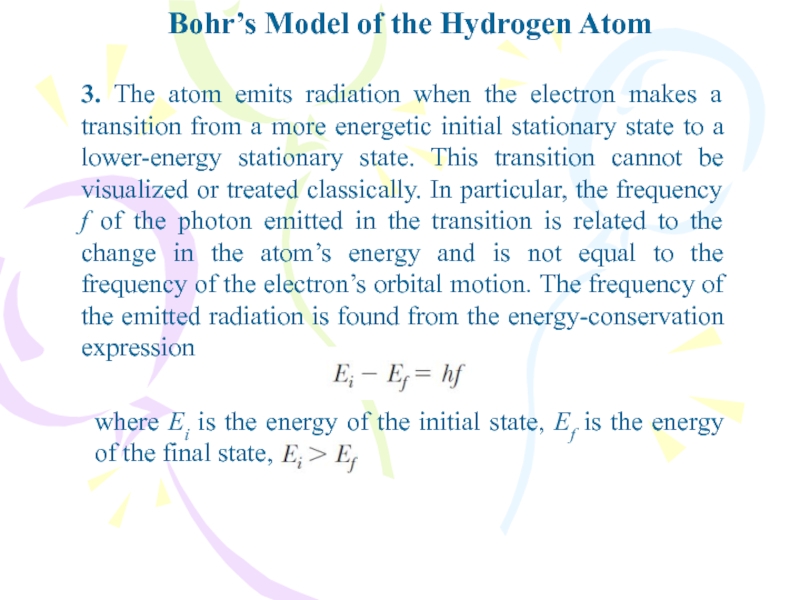
- 11. Bohr’s Model of the Hydrogen Atom3. The
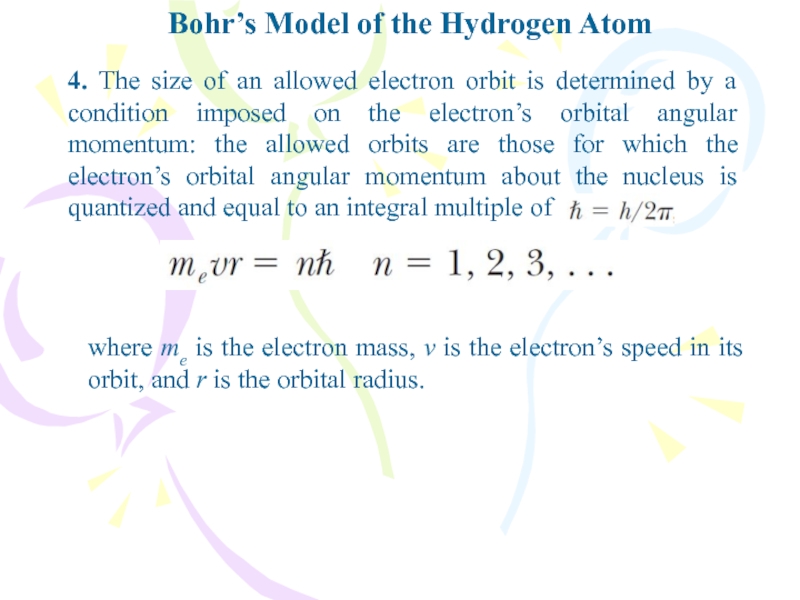
- 12. Bohr’s Model of the Hydrogen Atom4. The
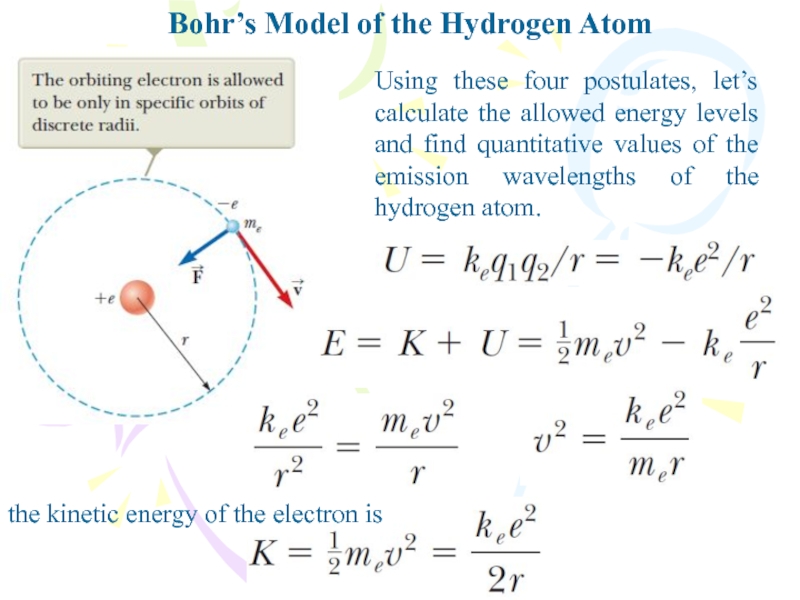
- 13. Bohr’s Model of the Hydrogen AtomUsing these
- 14. Bohr’s Model of the Hydrogen AtomThe following
- 15. Bohr’s Model of the Hydrogen AtomThe quantization
- 16. We can calculate the frequency of the
- 17. Bohr’s Model of the Hydrogen AtomSoon after
- 18. Bohr’s Model of the Hydrogen AtomBohr showed
- 19. Bohr’s Model of the Hydrogen AtomBohr’s Correspondence
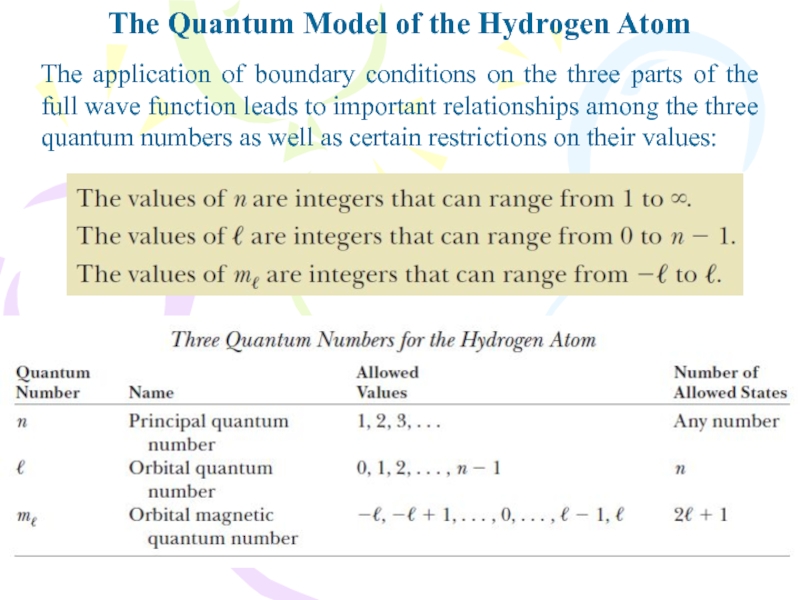
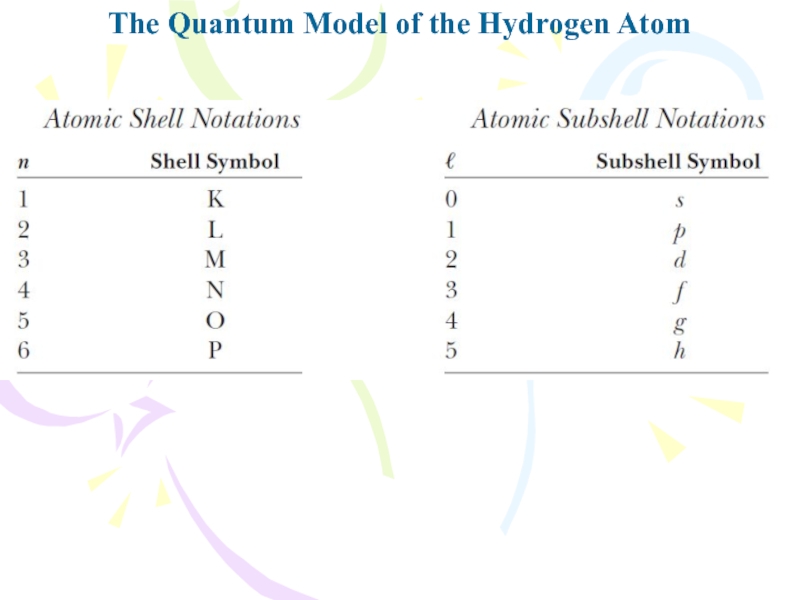
- 20. The Quantum Model of the Hydrogen AtomThe
- 21. The Quantum Model of the Hydrogen AtomThe
- 22. The Quantum Model of the Hydrogen AtomThe
- 23. The Quantum Model of the Hydrogen Atom
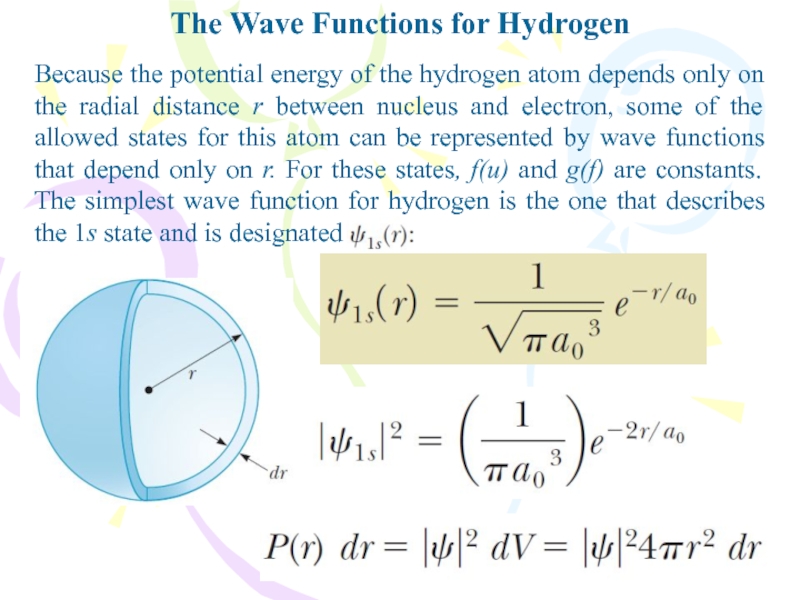
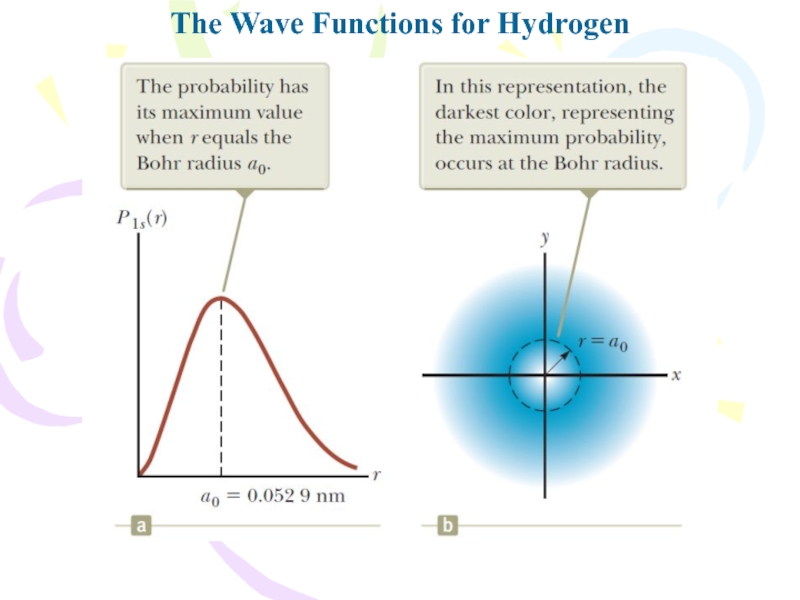
- 24. The Wave Functions for HydrogenBecause the potential
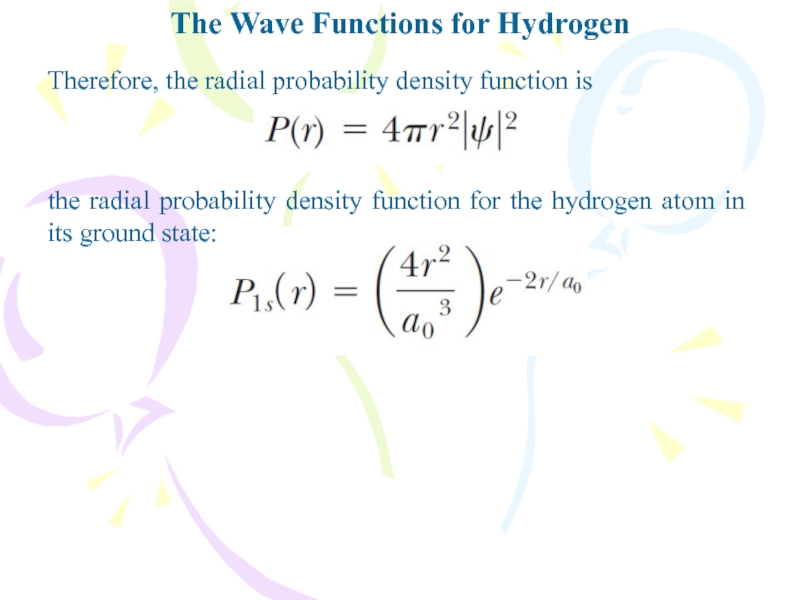
- 25. The Wave Functions for HydrogenTherefore, the radial
- 26. The Wave Functions for Hydrogen
- 27. Скачать презентанцию
Слайды и текст этой презентации
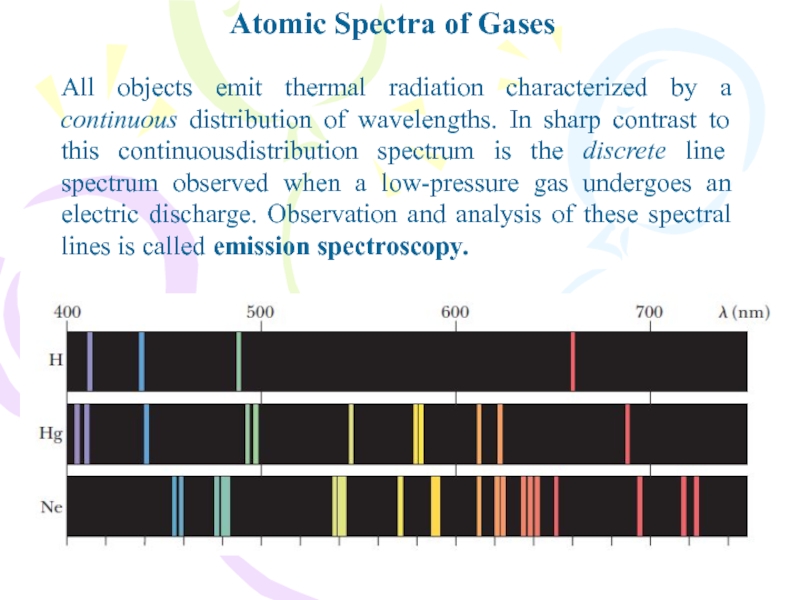
Слайд 2Atomic Spectra of Gases
All objects emit thermal radiation characterized by
a continuous distribution of wavelengths. In sharp contrast to this
continuousdistribution spectrum is the discrete line spectrum observed when a low-pressure gas undergoes an electric discharge. Observation and analysis of these spectral lines is called emission spectroscopy.Слайд 3Atomic Spectra of Gases
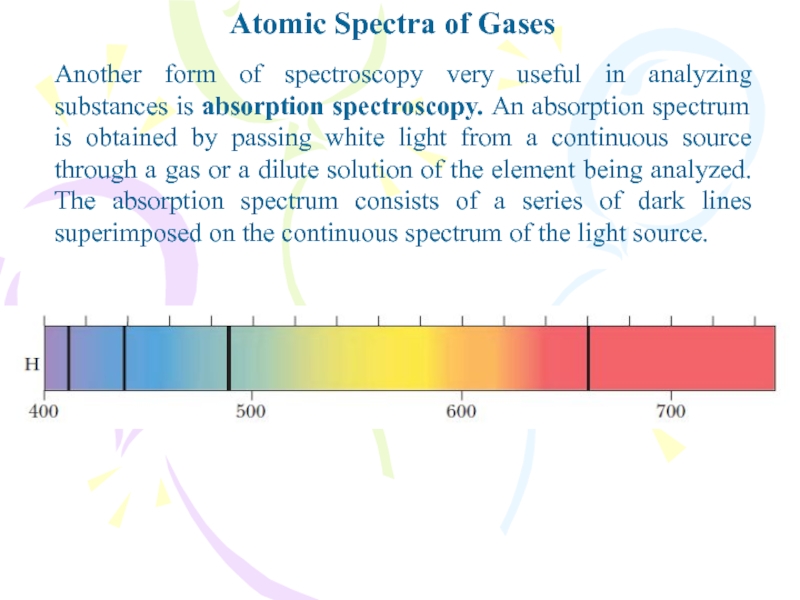
Another form of spectroscopy very useful in
analyzing substances is absorption spectroscopy. An absorption spectrum is obtained
by passing white light from a continuous source through a gas or a dilute solution of the element being analyzed. The absorption spectrum consists of a series of dark lines superimposed on the continuous spectrum of the light source.Слайд 4Atomic Spectra of Gases
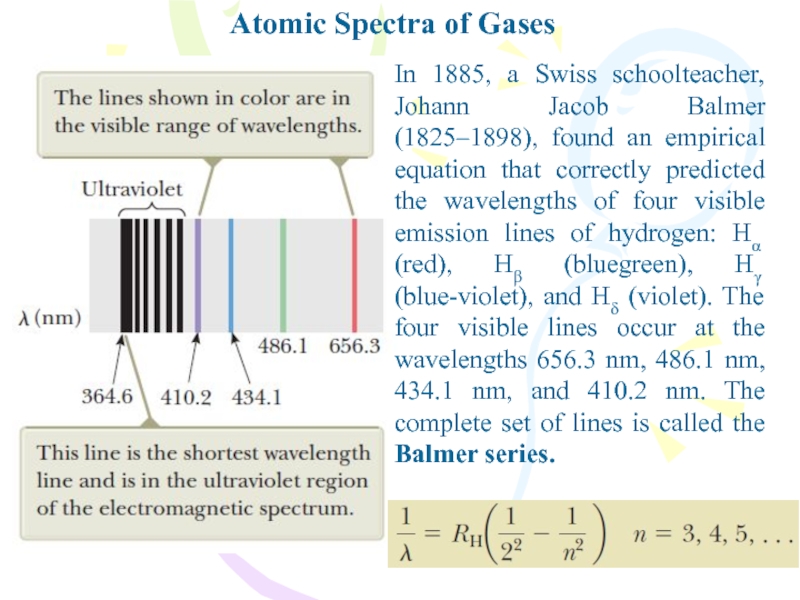
In 1885, a Swiss schoolteacher, Johann Jacob
Balmer (1825–1898), found an empirical equation that correctly predicted the
wavelengths of four visible emission lines of hydrogen: Hα (red), Hβ (bluegreen), Hγ (blue-violet), and Hδ (violet). The four visible lines occur at the wavelengths 656.3 nm, 486.1 nm, 434.1 nm, and 410.2 nm. The complete set of lines is called the Balmer series.Слайд 5Atomic Spectra of Gases
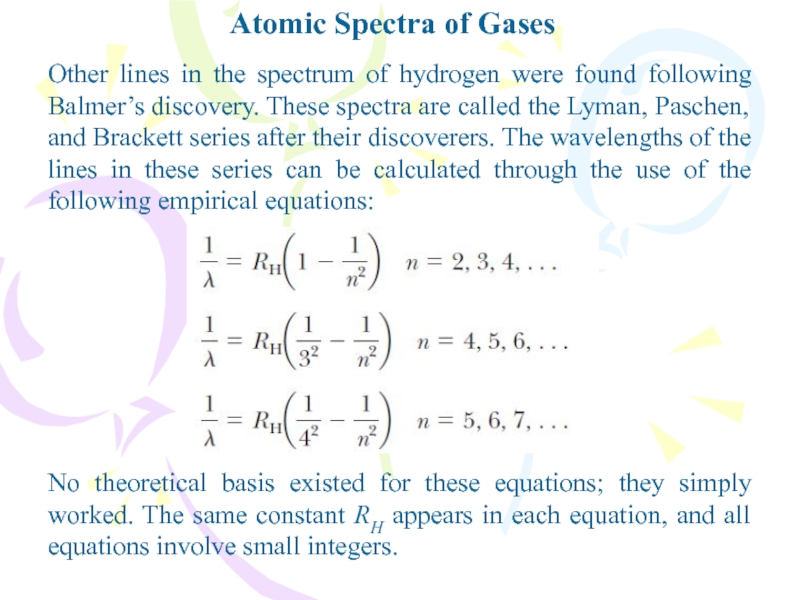
Other lines in the spectrum of hydrogen
were found following Balmer’s discovery. These spectra are called the
Lyman, Paschen, and Brackett series after their discoverers. The wavelengths of the lines in these series can be calculated through the use of the following empirical equations:No theoretical basis existed for these equations; they simply worked. The same constant RH appears in each equation, and all equations involve small integers.
Слайд 8Early Models of the Atom
Two basic difficulties exist with Rutherford’s
planetary model. As we previously an atom emits (and absorbs)
certain characteristic frequencies of electromagnetic radiation and no others, but the Rutherford model cannot explain this phenomenon. A second difficulty is that Rutherford’s electrons are undergoing a centripetal acceleration. According to Maxwell’s theory of electromagnetism, centripetally accelerated charges revolving with frequency f should radiate electromagnetic waves of frequency f. Unfortunately, this classical model leads to a prediction of self-destruction when applied to the atom. As the electron radiates, energy is carried away from the atom, the radius of the electron’s orbit steadily decreases, and its frequency of revolution increases. This process would lead to an ever-increasing frequency of emitted radiation and an ultimate collapse of the atom as the electron plunges into the nucleus .Слайд 9Bohr’s Model of the Hydrogen Atom
Bohr combined ideas from Planck’s
original quantum theory, Einstein’s concept of the photon, Rutherford’s planetary
model of the atom, and Newtonian mechanics to arrive at a semiclassical model based on some revolutionary ideas. The postulates of the Bohr theory as it applies to the hydrogen atom are as follows:1. The electron moves in circular orbits around the proton under the influence of the electric force of attraction as shown in Figure.
Слайд 10Bohr’s Model of the Hydrogen Atom
2. Only certain electron orbits
are stable. When in one of these stationary states, as
Bohr called them, the electron does not emit energy in the form of radiation, even though it is accelerating. Hence, the total energy of the atom remains constant and classical mechanics can be used to describe the electron’s motion. Bohr’s model claims that the centripetally accelerated electron does not continuously emit radiation, losing energy and eventually spiraling into the nucleus, as predicted by classical physics in the form of Rutherford’s planetary model.Слайд 11Bohr’s Model of the Hydrogen Atom
3. The atom emits radiation
when the electron makes a transition from a more energetic
initial stationary state to a lower-energy stationary state. This transition cannot be visualized or treated classically. In particular, the frequency f of the photon emitted in the transition is related to the change in the atom’s energy and is not equal to the frequency of the electron’s orbital motion. The frequency of the emitted radiation is found from the energy-conservation expressionwhere Ei is the energy of the initial state, Ef is the energy of the final state,
Слайд 12Bohr’s Model of the Hydrogen Atom
4. The size of an
allowed electron orbit is determined by a condition imposed on
the electron’s orbital angular momentum: the allowed orbits are those for which the electron’s orbital angular momentum about the nucleus is quantized and equal to an integral multiple ofwhere me is the electron mass, v is the electron’s speed in its orbit, and r is the orbital radius.
Слайд 13Bohr’s Model of the Hydrogen Atom
Using these four postulates, let’s
calculate the allowed energy levels and find quantitative values of
the emission wavelengths of the hydrogen atom.the kinetic energy of the electron is
Слайд 14Bohr’s Model of the Hydrogen Atom
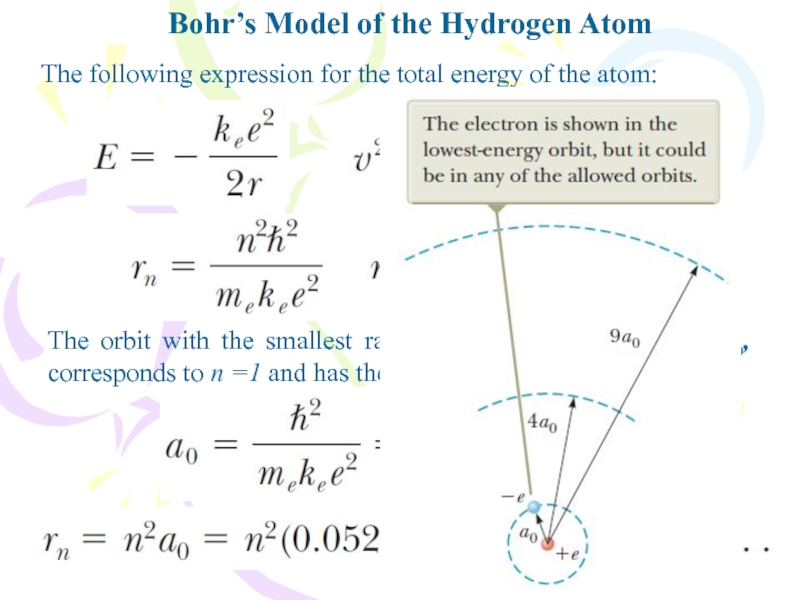
The following expression for the
total energy of the atom:
The orbit with the smallest radius,
called the Bohr radius a0, corresponds to n =1 and has the valueСлайд 15Bohr’s Model of the Hydrogen Atom
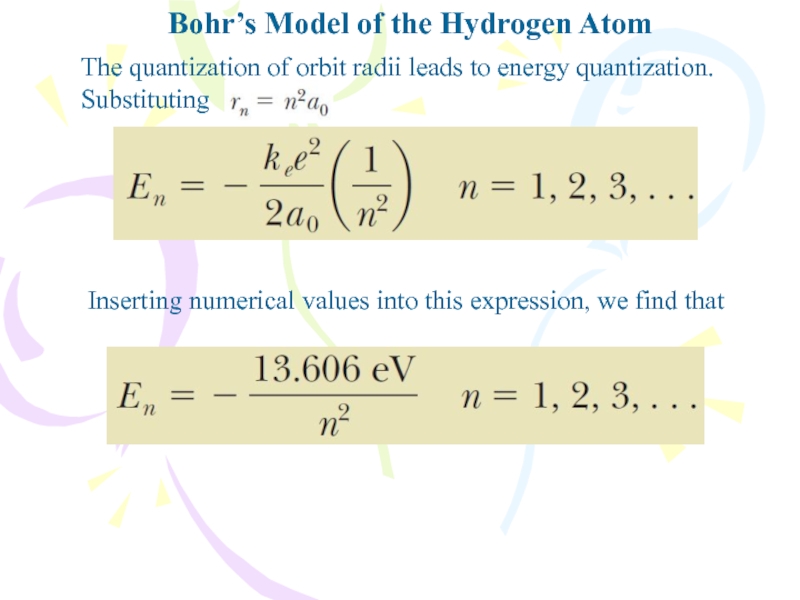
The quantization of orbit radii
leads to energy quantization. Substituting
Inserting numerical values into this expression,
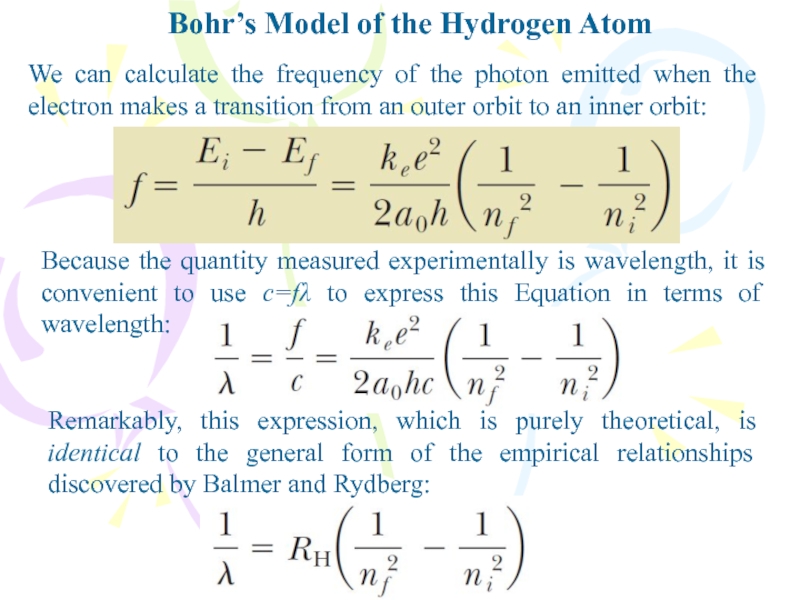
we find thatСлайд 16We can calculate the frequency of the photon emitted when
the electron makes a transition from an outer orbit to
an inner orbit:Bohr’s Model of the Hydrogen Atom
Because the quantity measured experimentally is wavelength, it is convenient to use c=fλ to express this Equation in terms of wavelength:
Remarkably, this expression, which is purely theoretical, is identical to the general form of the empirical relationships discovered by Balmer and Rydberg:
Слайд 17Bohr’s Model of the Hydrogen Atom
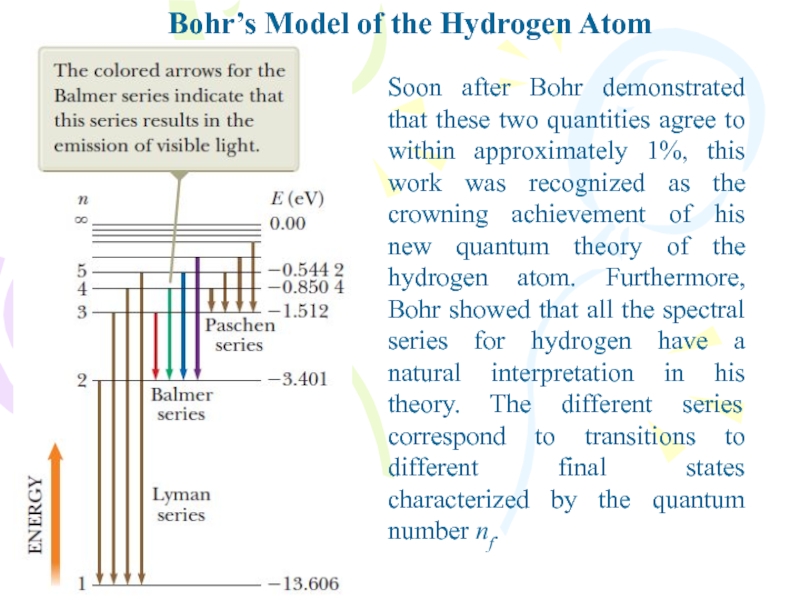
Soon after Bohr demonstrated that
these two quantities agree to within approximately 1%, this work
was recognized as the crowning achievement of his new quantum theory of the hydrogen atom. Furthermore, Bohr showed that all the spectral series for hydrogen have a natural interpretation in his theory. The different series correspond to transitions to different final states characterized by the quantum number nf.Слайд 18Bohr’s Model of the Hydrogen Atom
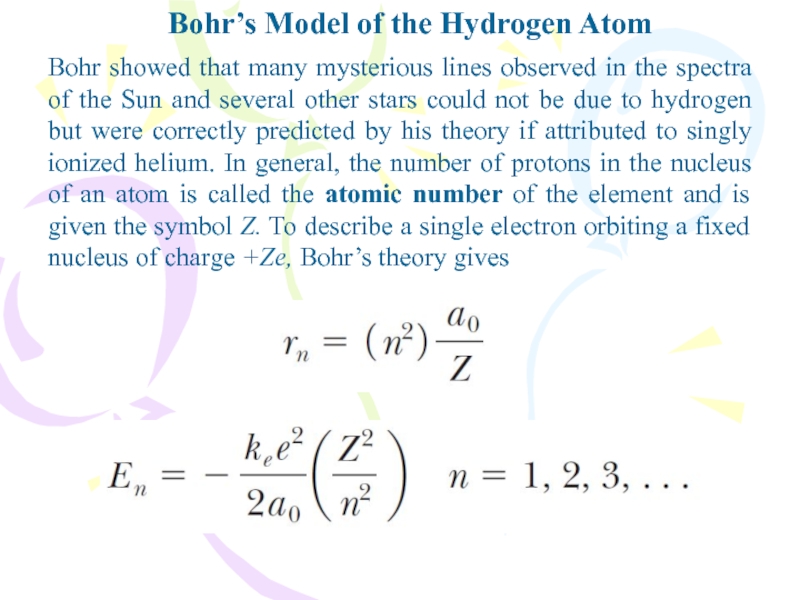
Bohr showed that many mysterious
lines observed in the spectra of the Sun and several
other stars could not be due to hydrogen but were correctly predicted by his theory if attributed to singly ionized helium. In general, the number of protons in the nucleus of an atom is called the atomic number of the element and is given the symbol Z. To describe a single electron orbiting a fixed nucleus of charge +Ze, Bohr’s theory givesСлайд 19Bohr’s Model of the Hydrogen Atom
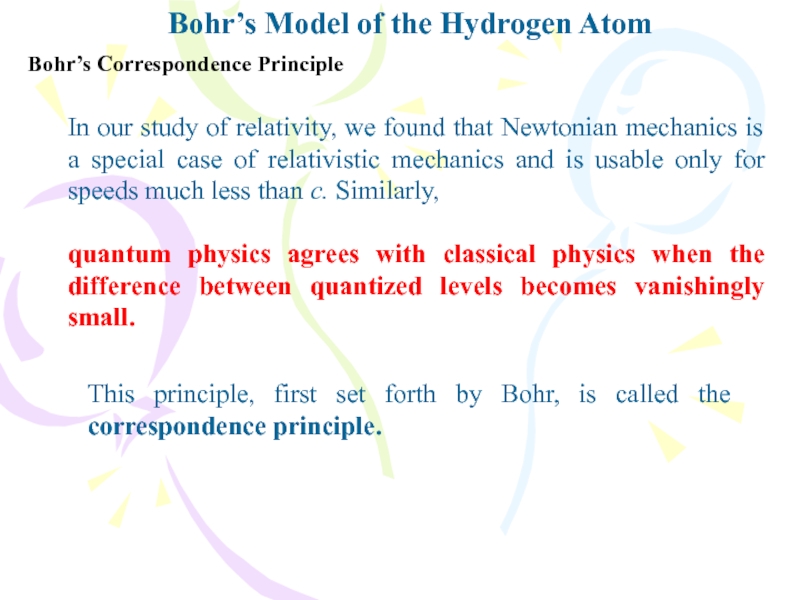
Bohr’s Correspondence Principle
In our study
of relativity, we found that Newtonian mechanics is a special
case of relativistic mechanics and is usable only for speeds much less than c. Similarly,quantum physics agrees with classical physics when the difference between quantized levels becomes vanishingly small.
This principle, first set forth by Bohr, is called the correspondence principle.
Слайд 20The Quantum Model of the Hydrogen Atom
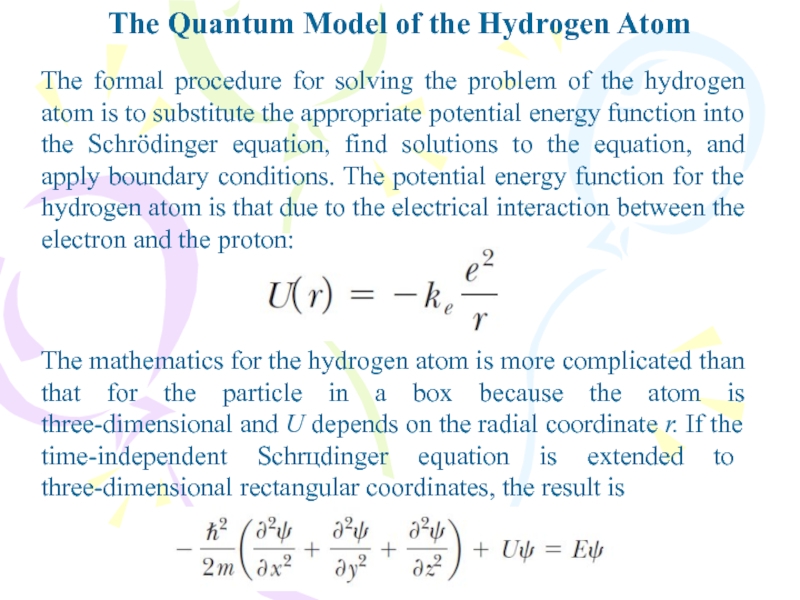
The formal procedure for
solving the problem of the hydrogen atom is to substitute
the appropriate potential energy function into the Schrӧdinger equation, find solutions to the equation, and apply boundary conditions. The potential energy function for the hydrogen atom is that due to the electrical interaction between the electron and the proton:The mathematics for the hydrogen atom is more complicated than that for the particle in a box because the atom is three-dimensional and U depends on the radial coordinate r. If the time-independent Schrцdinger equation is extended to three-dimensional rectangular coordinates, the result is
Слайд 21The Quantum Model of the Hydrogen Atom
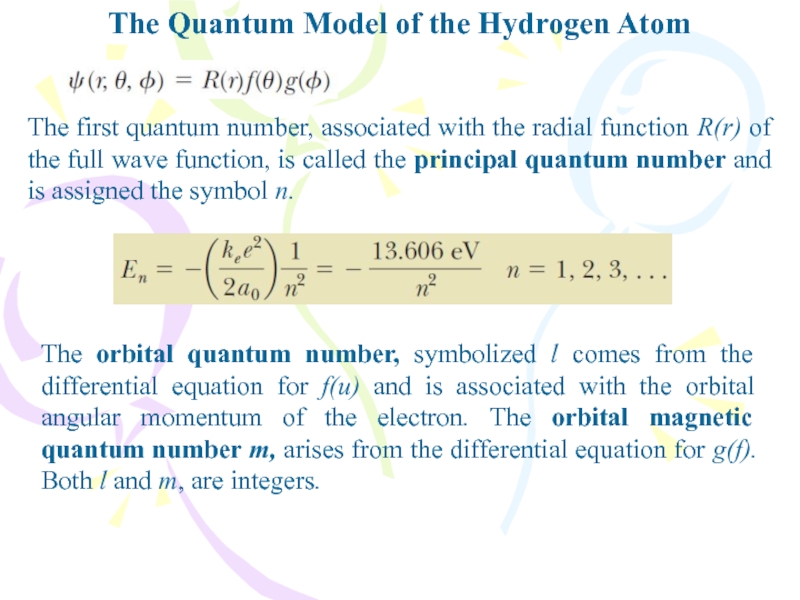
The first quantum number,
associated with the radial function R(r) of the full wave
function, is called the principal quantum number and is assigned the symbol n.The orbital quantum number, symbolized l comes from the differential equation for f(u) and is associated with the orbital angular momentum of the electron. The orbital magnetic quantum number m, arises from the differential equation for g(f). Both l and m, are integers.