Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Биологическое окисление
Содержание
- 1. Биологическое окисление
- 2. *СодержаниеБиоэнергетика: История развитияБиологическое окислениеЦТК (Цикл Кребса)
- 3. *БиоэнергетикаТермин введен лауреатом Нобелевской премии Альбертом Сент-Дьерди
- 4. *История учения о БОАнтичные авторы: Учение о
- 5. *Antoine Lavoisier В конце XVIII в. A.
- 6. *Теория активации кислородаВ 1840 Ф. Шёнбайн открыл
- 7. *Критическая оценка теории Баха-ЭнглераНе была найдена
- 8. *Теория Палладина-Виланда1903 – Н. Бор, создал теорию
- 9. *Хромогены и гистогематиныПереносчики электронов были названы хромогенами
- 10. *Реакции переноса электронов от донора к акцептору.Редокс
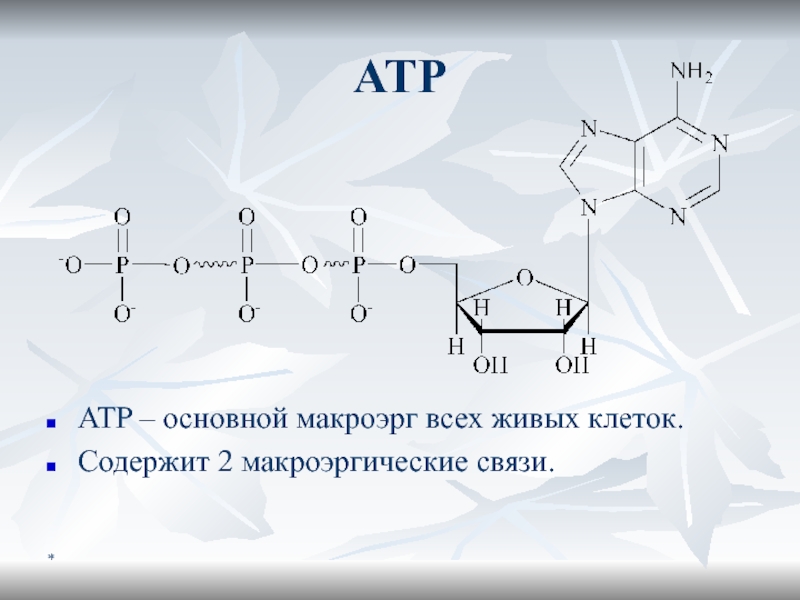
- 11. *ATPATP – основной макроэрг всех живых клеток.Содержит 2 макроэргические связи.
- 12. *Maкроэргичность ATФОтрицательно заряженный «хвост» создает сильное отталкиваниеATФ4-
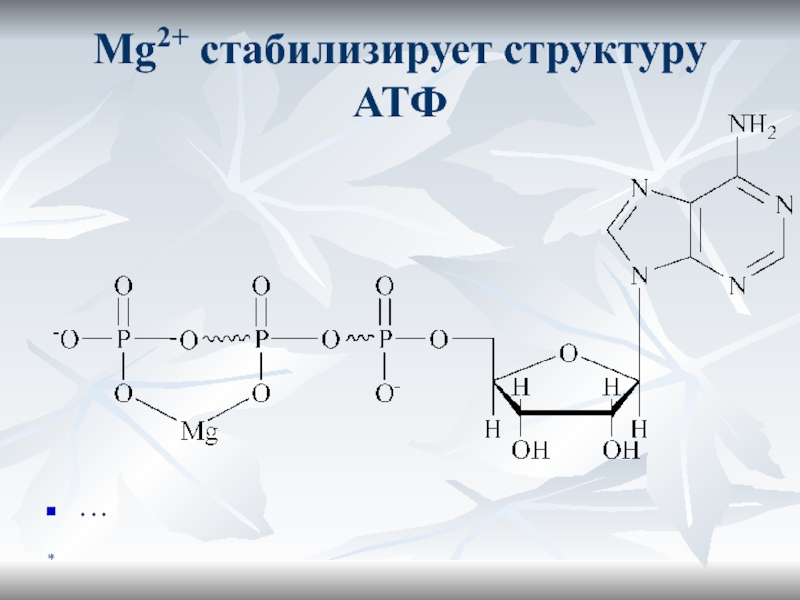
- 13. *Mg2+ стабилизирует структуру АТФ …
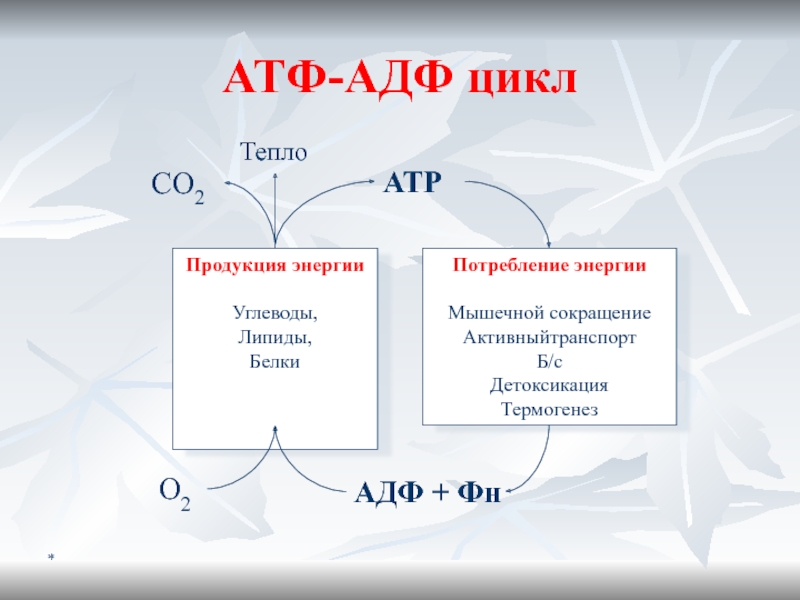
- 14. *ATФ-AДФ цикл
- 15. *
- 16. *Образование субстратов БОСтадия I: Б, Ж, У
- 17. *Stages 1 and 2
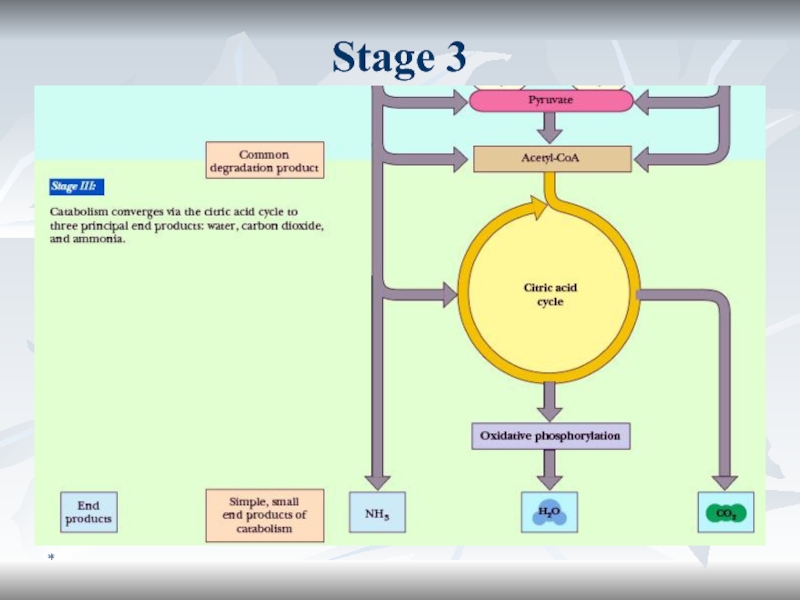
- 18. *Stage 3
- 19. *Ферменты и коферменты БООксидоредуктазыоксидазыДГПиризин-зависимые (NAD+, NADH+)Флавин-зависимые (FMN, FAD)ГидропероксидазыОкигеназы
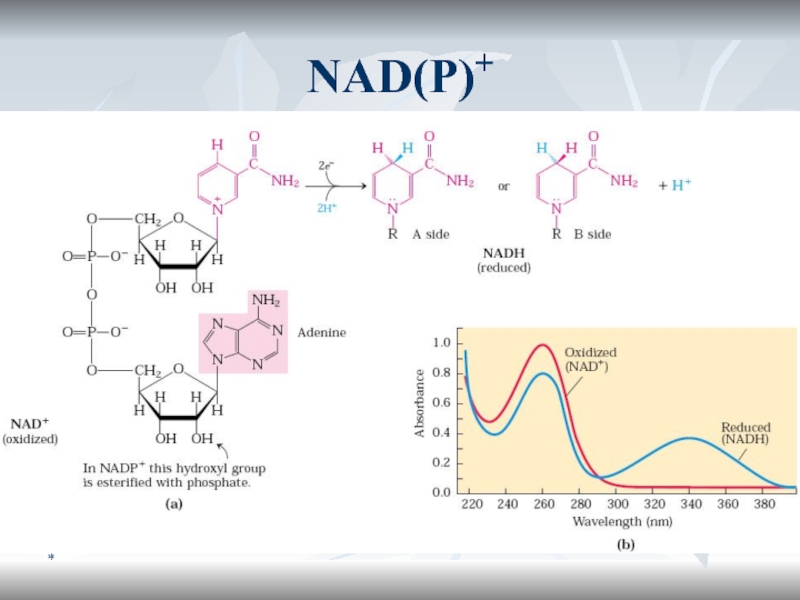
- 20. *NAD(P)+
- 21. *FAD, FMN
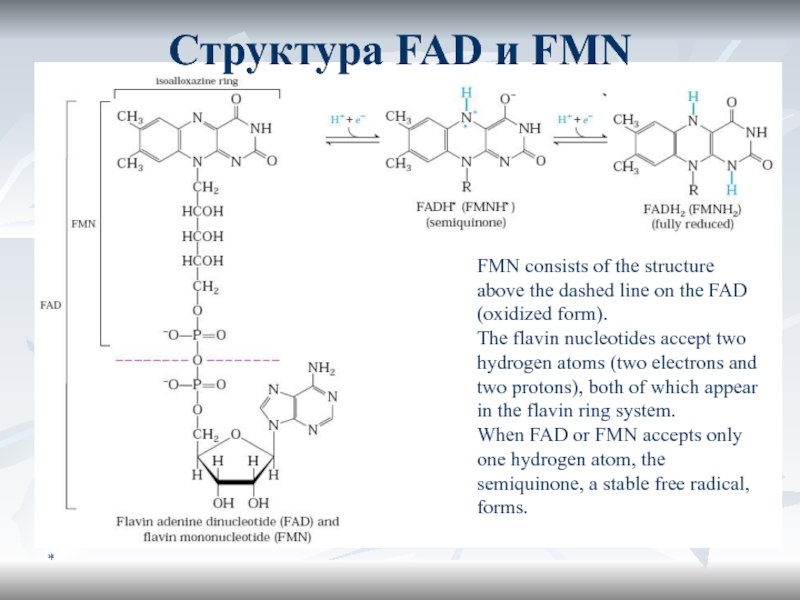
- 22. *FMN consists of the structure above the
- 23. *Мх: локализацияMх: цилиндр ∅ 0.5 - 1.0
- 24. *Общий план строения МхВ печени 67% всего
- 25. *Progress in Studying MitochondriaReal progress in understanding
- 26. *Internal structure of a mitochondrionThe principal
- 27. *The Comparative Characteristics of Mitochondrial MembranesOuter
- 28. *Membrane Composition: Lipid fractionsInner membrane contains
- 29. *Tricarboxylic Acid CycleA time-lapse photograph of a
- 30. *Krebs’ CycleHans Adolf Krebs, 1937.TCA is the
- 31. *Role of TCAEnergetic. 1 TCA turn =
- 32. *Plastic Role of TCA
- 33. *Regulatory Role of TCA: “Krebs’ bicycle”TCAUrea CycleaspartatefumarateOANH3, CO2UreaAcetyl-KoA
- 34. *At the level of entry of substrates
- 35. *Regulation of the TCA Cycle (cont’d)At the
- 36. *Inhibitors of Krebs Cycle
- 37. 2 Tissue respiration. Oxidative phosphorylation. Microsomal oxidation and peroxidationAlexander KOVAL PhD, senior lecturer
- 38. *ContentThe Ways of Oxygen Consumption in the
- 39. *Introduction
- 40. *The Ways of Oxygen Consumption in the
- 41. *Biologic Oxidation (BO)Oxidation is the removal of
- 42. *Biomedical Importance of BOO2 is incorporated into
- 43. *Energy Conversion: MitochondriaAfter the cytosolic stage of
- 44. *Chemiosmotic CouplingThe common pathway used by mitochondria,
- 45. *Electron Transporting Chain, ETC (1/2)The mitochondria contain
- 46. *Electron Transporting Chain (ETC) (2/2)
- 47. *ETC functionsIt is the final common pathway
- 48. *ETC Complexes: Overview
- 49. *Complex I (NADH-CoQ reductase)Contains:FMNFeS centres (22-24 iron-sulfur
- 50. *Coenzyme Q (CoQ) or UbiquinoneCoQ is a
- 51. *CoQ10CoQ is oxidized by cytochromes, it is a collection point of electrons from several flavoprotein dehydrogenases.
- 52. *Complex II (Succinate-CoQ reductase)Complex II contains
- 53. *Complex II and IIICoQ accepts electrons from
- 54. *Q-cycle (1/2)The electron transfer pathway following oxidation
- 55. *Q-cycle (2/2)The pathway following oxidation of a second UQH2.
- 56. *Complex IV: Cytochrome c OxidaseComplex IV is
- 57. *H+-ATPaseIon gradient across a membrane is a
- 58. *ATP/ADP translocaseOutward transport of ATP (via the ATP/ADP translocase) is favored by the membrane electrochemical potential.
- 59. *Respiratory Chain Functioning
- 60. *Functional scheme of ETCThere are 3 cycles
- 61. *Inhibitors of Oxidative Phosphorylation
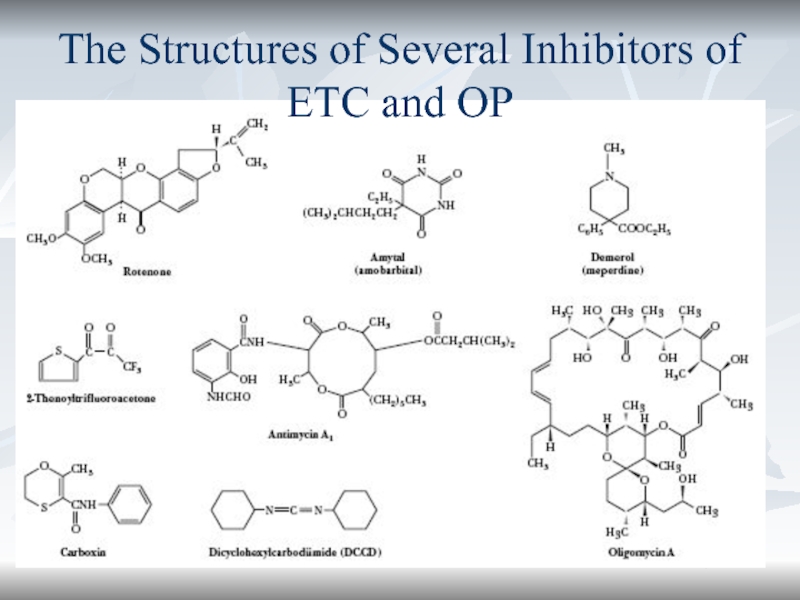
- 62. *The Structures of Several Inhibitors of ETC and OP
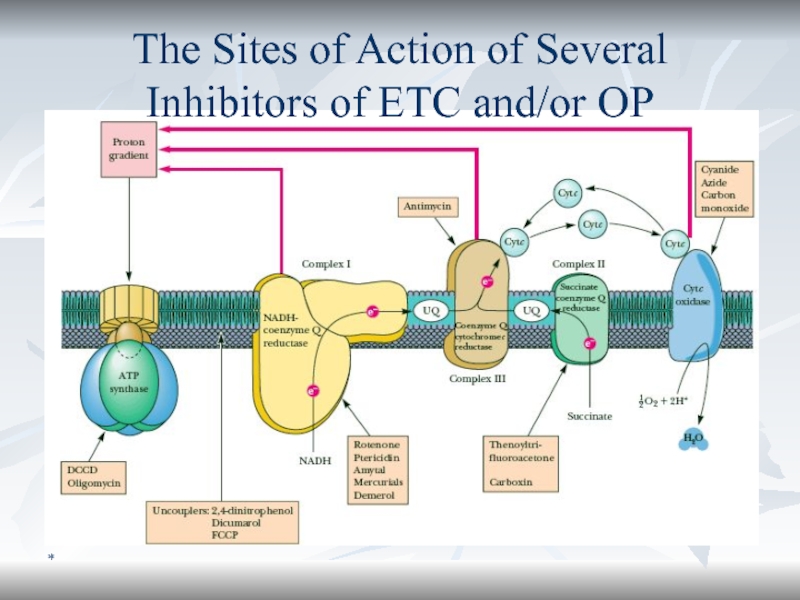
- 63. *The Sites of Action of Several Inhibitors of ETC and/or OP
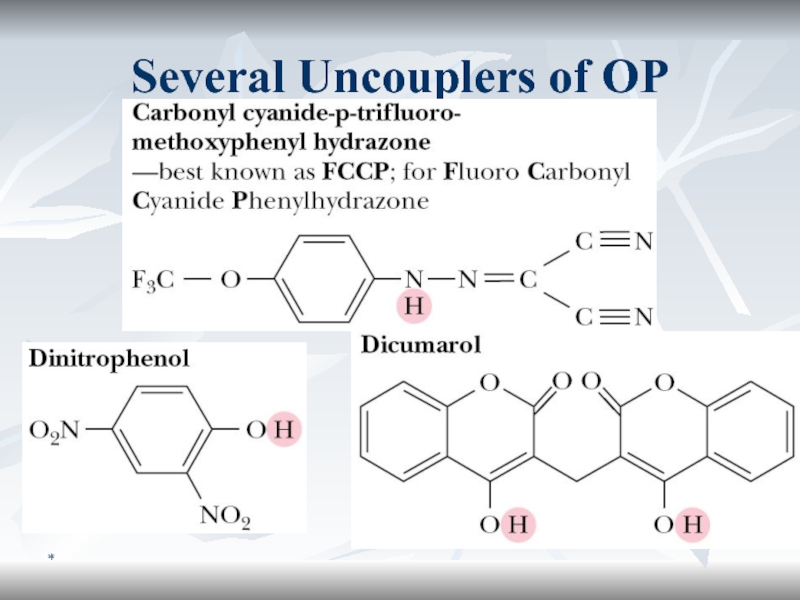
- 64. *Several Uncouplers of OP
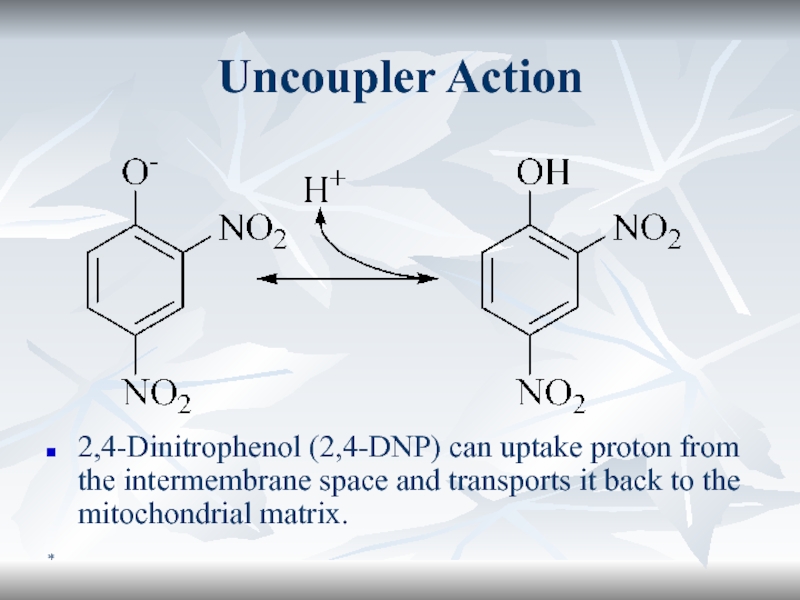
- 65. *Uncoupler Action2,4-Dinitrophenol (2,4-DNP) can uptake proton from
- 66. *2,4-DNP decreases ΔμH+Intermembrane spaceMitochondrial matrixH+H+H+H+H+H+H+H+H+H+H+H+H+H+H+H+H+H+H+H+H+H+H+H+H+H+25:1 H+
- 67. *Endogenous Uncouplers Enable Organisms To Generate HeatCertain
- 68. *P/O RatioElectrons that enter the chain from
- 69. *Disorders of Mitochondrial Oxidative PhosphorylationMitochondria contain DNA
- 70. *Clinical Manifestation and Treatment of Mito DisordersManifestationMuscle
- 71. *Some Mitochondrial DiseasesThe names of mitochondrial diseases
- 72. *LHONLHON is a hereditary disease that often
- 73. *MERRF, MELAS et al.The most frequent (80
- 74. *Can Mitochondrial Diseases be Treated? Attempts are
- 75. *Cytochromes P450 are monooxygenases important for the
- 76. *Cytochrome b5In liver microsomes, cytochromes P450 are
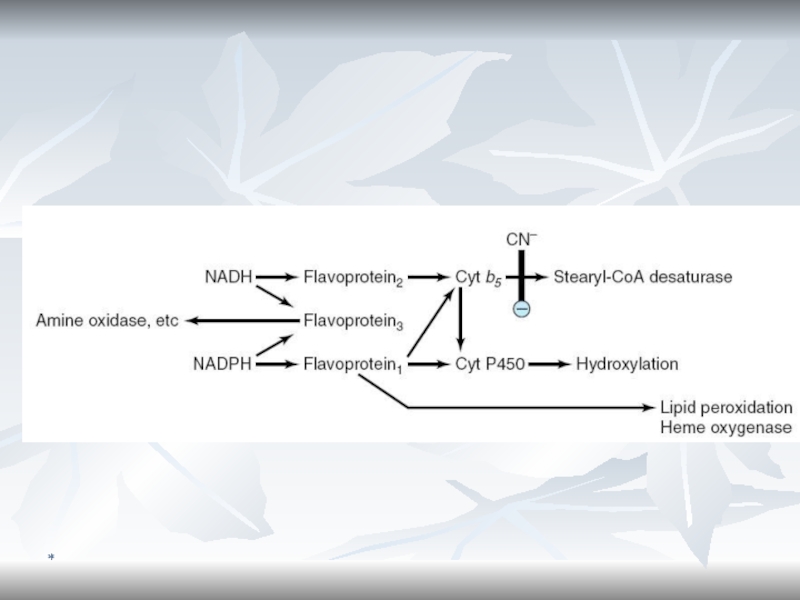
- 77. *Monooxygenase System (Microsomal Oxidation)The substrate can be
- 78. *Functioning of Microsomal Respiratory ChainCyt P450RHR-OHO2H2OCyt b5FMNNADP + H+NADP + H+NADP+ e-e-
- 79. *Microsomal Oxidation and Cytochrome P450
- 80. *
- 81. *
- 82. *
- 83. *Conclusion
- 84. *
- 85. Скачать презентанцию
Слайды и текст этой презентации
Слайд 1*
Биологическое окисление 1
Лектор
д.м.н., проф. Грицук А. И.
зав. кафедрой биохимии
Гомельского государственного медицинского университета
Слайд 3*
Биоэнергетика
Термин введен лауреатом Нобелевской премии Альбертом Сент-Дьерди
Биоэнергетика - раздел
биохимии изучающей механизмы и пути превращения энергии в живых системах
Слайд 4*
История учения о БО
Античные авторы: Учение о 4 стихиях обсуждали
роль воздуха в БО Платон (воздух необходим для поддержания внутреннего
огня) «Могущественные боги, предоставив нам, немощным пищу, разделили тело наше каналами, чтобы оно могло орошаться как бы из некого идущего сверху потока… Получив орошение и освежение, оно имеет возможность питаться и жить. Ибо когда воздух входит внутрь и выходит вон, то и соединенный с ним внутренний огонь охватывает пищу и питье, расплавляя их, разлагает на мелкие частицы и затем приносит к жилам» ПлатонАристотель (воздух для охлаждения внутренностей и крови)
XVII в. Georg Ernst Stahl создал теорию флогистона.
1770 годы . Carl Scheele и Joseph Priestley открыли бесфлогистонный воздух, который впоследствии А. Лавуазье назвал кислородом
Слайд 5*
Antoine Lavoisier
В конце XVIII в. A. Лавуазье ввел в
химические исследования количественный метод и создал кислородную теорию горения
A.
Лавуазье установил сходство горения и дыхания на основании количественного анализа конечных продуктовСлайд 6*
Теория активации кислорода
В 1840 Ф. Шёнбайн открыл озон, более активную
форму О2
В конце XIX в. – почти одновременно A.Н.
Бах (Россия) и К. Энглер (Германия) создали теорию активации кислорода :Гипотетическая форма озона
Слайд 7*
Критическая оценка теории
Баха-Энглера
Не была найдена высокая активность оксигеназ в
живых организмах.
Не была найдена высокая концентрация H2O2 в живых организмах.
Были
обнаружены ферменты деградации H2O2 в живых организмах (каталаза и пероксидаза).Слайд 8*
Теория Палладина-Виланда
1903 – Н. Бор, создал теорию строения атома (ядро,
электроны).
Возникло иное представление об О-В процессах (бескислородное окисление)
1912 –В.И.
Палладин и Г. Виланд создали теорию «активации водорода», предполагающую наличие 2-х стадий: Анаэробная стадия: SH2 + R = S + RH2 (см рис.)
Аэробная стадия : RH2 + 1/2 O2 ? R + H2O.
Слайд 9*
Хромогены и гистогематины
Переносчики электронов были названы хромогенами из-за окраски зависящей
от О-В состояния
В качестве хромогенов позднее идентифицированы коферменты FMN, FAD,
NAD+, NADP+.1925 –открыты гистогематины (цитохромы)
1932 – акад В.А. Энгельгардт открыл сопряженное окислительное фосфорилирование (ADP + Pi ? ATP).
Слайд 10*
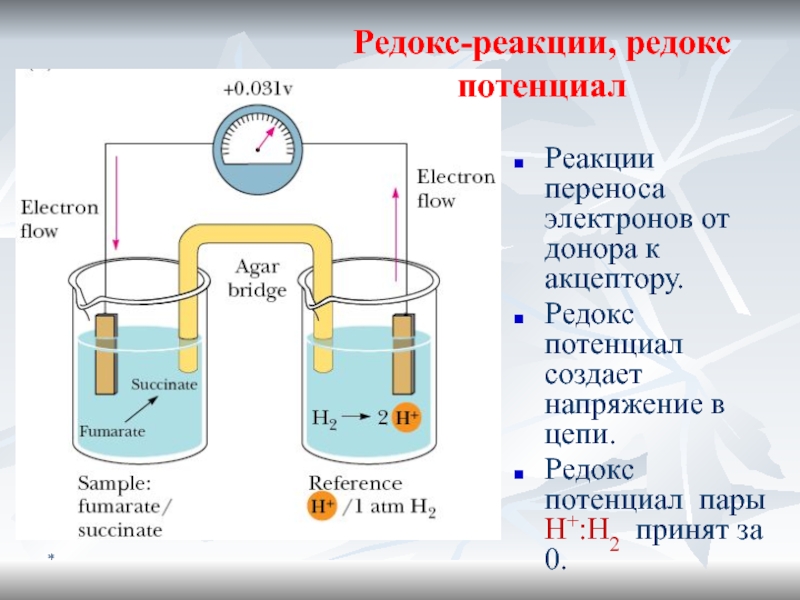
Реакции переноса электронов от донора к акцептору.
Редокс потенциал создает напряжение
в цепи.
Редокс потенциал пары H+:H2 принят за 0.
Редокс-реакции, редокс потенциал
Слайд 12*
Maкроэргичность ATФ
Отрицательно заряженный «хвост» создает сильное отталкивание
ATФ4- ? ADФ3- +
Фн2- + H+
[ATФ4-] = [ADФ3-] = [] = [H+] =
10-3 MЕсли [H+] будет 10-3 M, pH=3. Но pH=7, значит [H+] =10-7, и хим равновесие сильно сдвинуто влево
ADФ3- и Фн2- резонансные гибриды, для которых характерна высокая устойчивость
В результате
G(ATФ4-) >> G(ADФ3-) + G(Фн2-) + G(H+)
Слайд 16*
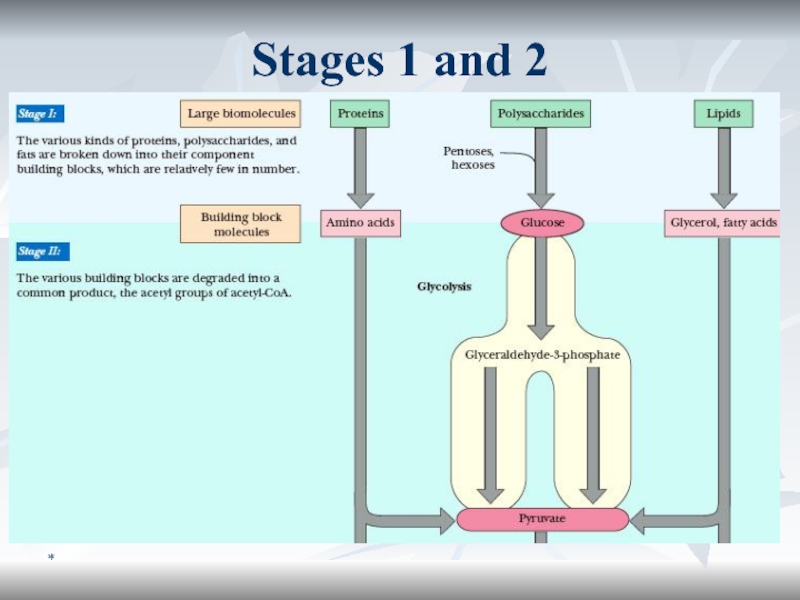
Образование субстратов БО
Стадия I:
Б, Ж, У расщепляются на относительно
небольшое кол-во мономеров
Стадия II:
Полученные строительные блоки превращаются в
унифицированные метаболиты (ПВК, ацетил КоА и др.). Стадия III:
Катаболиз унифицированныех метаболитов до СО2 и Н2О с выделением полезной конвертируемой энергии
Слайд 19*
Ферменты и коферменты БО
Оксидоредуктазы
оксидазы
ДГ
Пиризин-зависимые (NAD+, NADH+)
Флавин-зависимые (FMN, FAD)
Гидропероксидазы
Окигеназы
Слайд 22*
FMN consists of the structure above the dashed line on
the FAD (oxidized form).
The flavin nucleotides accept two hydrogen
atoms (two electrons and two protons), both of which appear in the flavin ring system. When FAD or FMN accepts only one hydrogen atom, the semiquinone, a stable free radical, forms.
Структура FAD и FMN
Слайд 23*
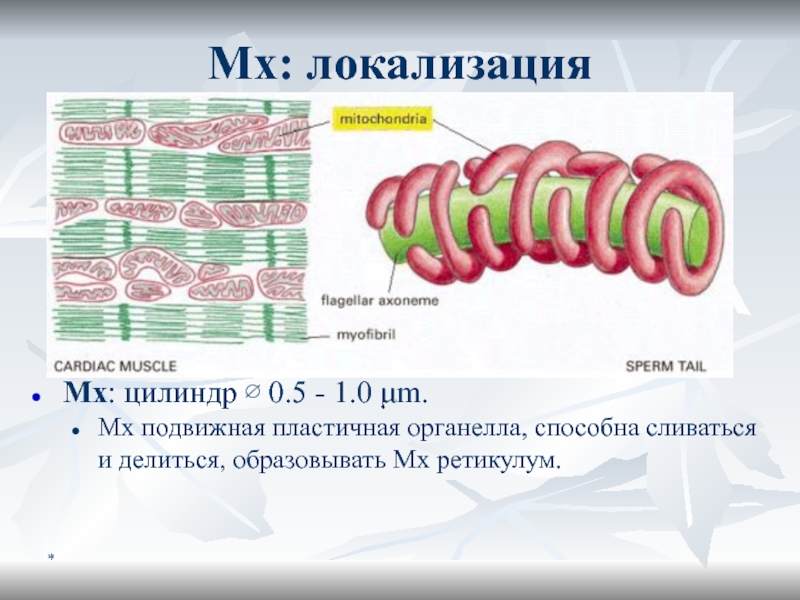
Мх: локализация
Mх: цилиндр ∅ 0.5 - 1.0 μm.
Мх подвижная пластичная
органелла, способна сливаться и делиться, образовывать Мх ретикулум.
Слайд 24*
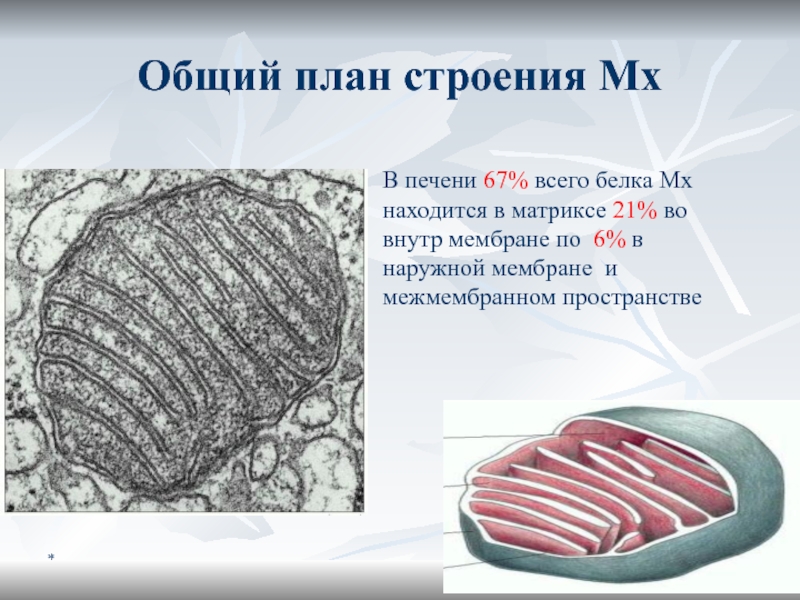
Общий план строения Мх
В печени 67% всего белка Мх находится
в матриксе 21% во внутр мембране по 6% в наружной
мембране и межмембранном пространствеСлайд 25*
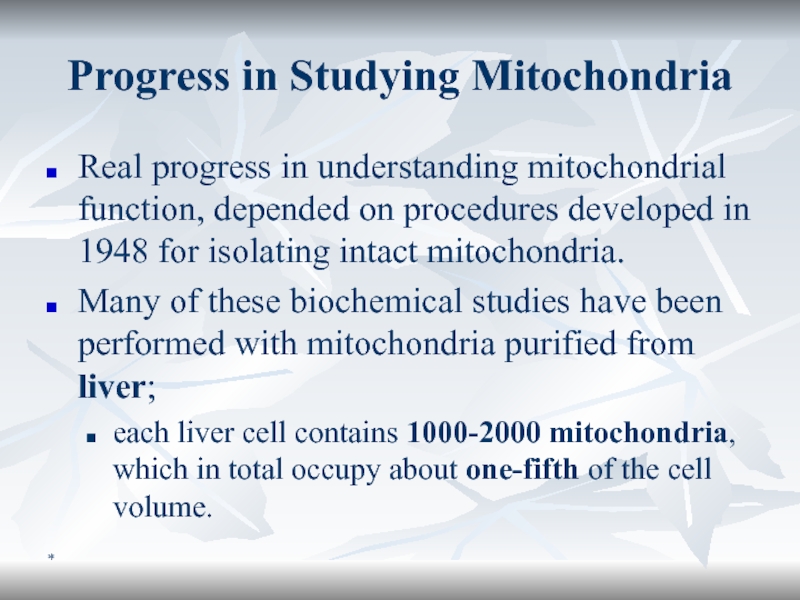
Progress in Studying Mitochondria
Real progress in understanding mitochondrial function, depended
on procedures developed in 1948 for isolating intact mitochondria.
Many
of these biochemical studies have been performed with mitochondria purified from liver; each liver cell contains 1000-2000 mitochondria, which in total occupy about one-fifth of the cell volume.
Слайд 26*
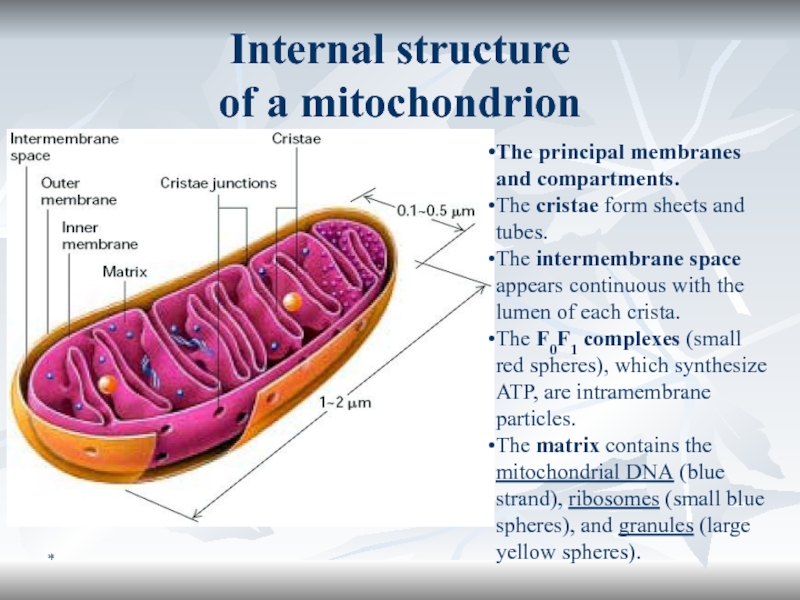
Internal structure
of a mitochondrion
The principal membranes and compartments.
The
cristae form sheets and tubes.
The intermembrane space appears continuous
with the lumen of each crista. The F0F1 complexes (small red spheres), which synthesize ATP, are intramembrane particles.
The matrix contains the mitochondrial DNA (blue strand), ribosomes (small blue spheres), and granules (large yellow spheres).
Слайд 27*
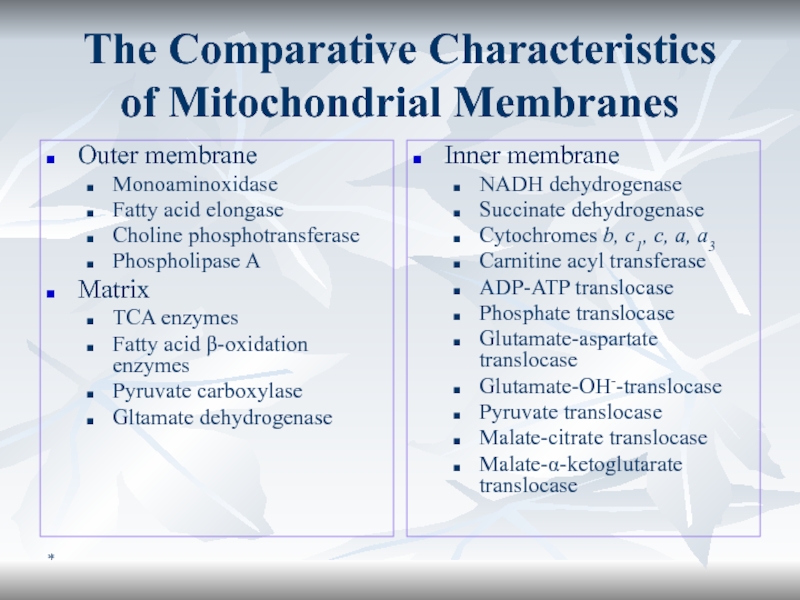
The Comparative Characteristics
of Mitochondrial Membranes
Outer membrane
Monoaminoxidase
Fatty acid elongase
Choline phosphotransferase
Phospholipase
A
Matrix
TCA enzymes
Fatty acid β-oxidation enzymes
Pyruvate carboxylase
Gltamate dehydrogenase
Inner membrane
NADH dehydrogenase
Succinate dehydrogenase
Cytochromes
b, c1, c, a, a3Carnitine acyl transferase
ADP-ATP translocase
Phosphate translocase
Glutamate-aspartate translocase
Glutamate-OH--translocase
Pyruvate translocase
Malate-citrate translocase
Malate-α-ketoglutarate translocase
Слайд 28*
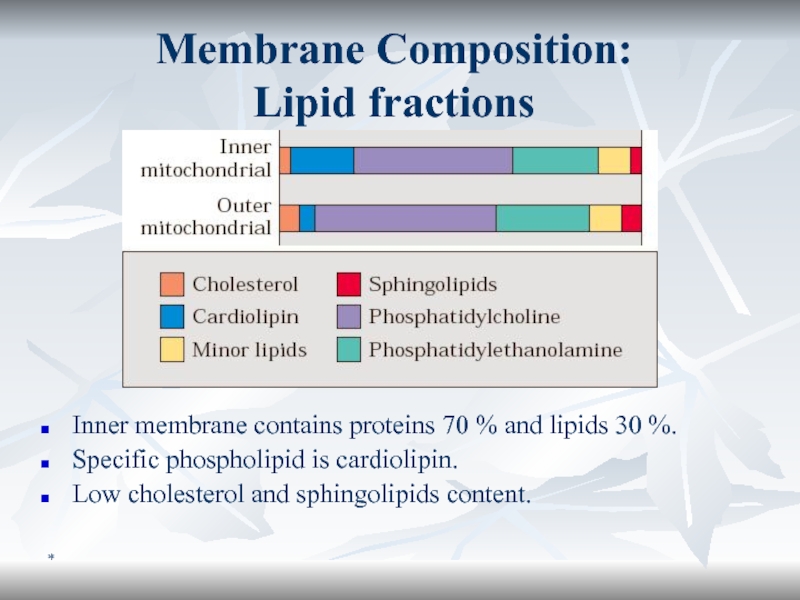
Membrane Composition:
Lipid fractions
Inner membrane contains proteins 70 % and
lipids 30 %.
Specific phospholipid is cardiolipin.
Low cholesterol and sphingolipids content.
Слайд 29*
Tricarboxylic Acid Cycle
A time-lapse photograph of a ferris wheel at
night. Aerobic cells use a metabolic wheel—the tricarboxylic acid cycle—to
generate energy by acetyl-CoA oxidation.(Ferns Wheel, DelMar Fair © Corbis/Richard Cummins)
Слайд 30*
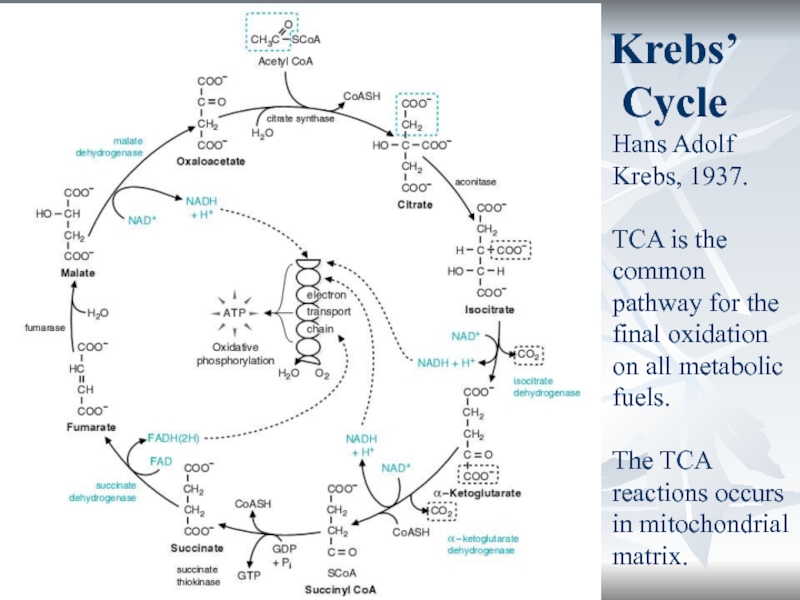
Krebs’ Cycle
Hans Adolf Krebs, 1937.
TCA is the common pathway for
the final oxidation on all metabolic fuels.
The TCA reactions occurs
in mitochondrial matrix.Слайд 31*
Role of TCA
Energetic.
1 TCA turn = 12 ATP.
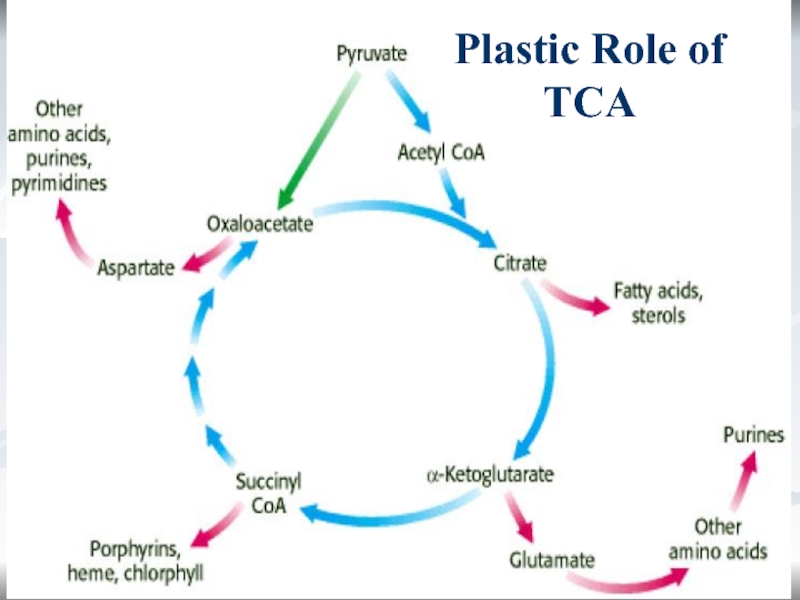
Plastic.
α-KG
? glu.
OA ? asp.
Succinyl-CoA ? heme.
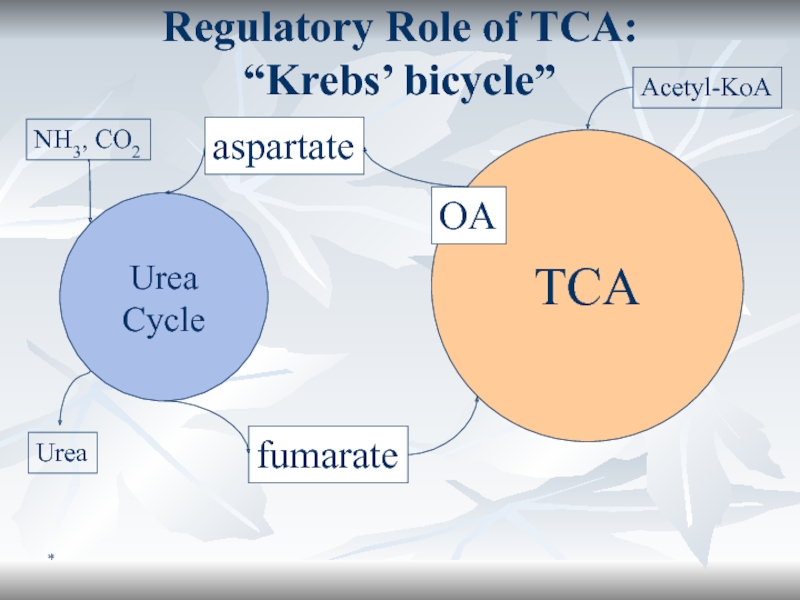
Regulatory.
Urea cycle (formation
of urea in liver) depends on TCA.Слайд 33*
Regulatory Role of TCA:
“Krebs’ bicycle”
TCA
Urea Cycle
aspartate
fumarate
OA
NH3, CO2
Urea
Acetyl-KoA
Слайд 34*
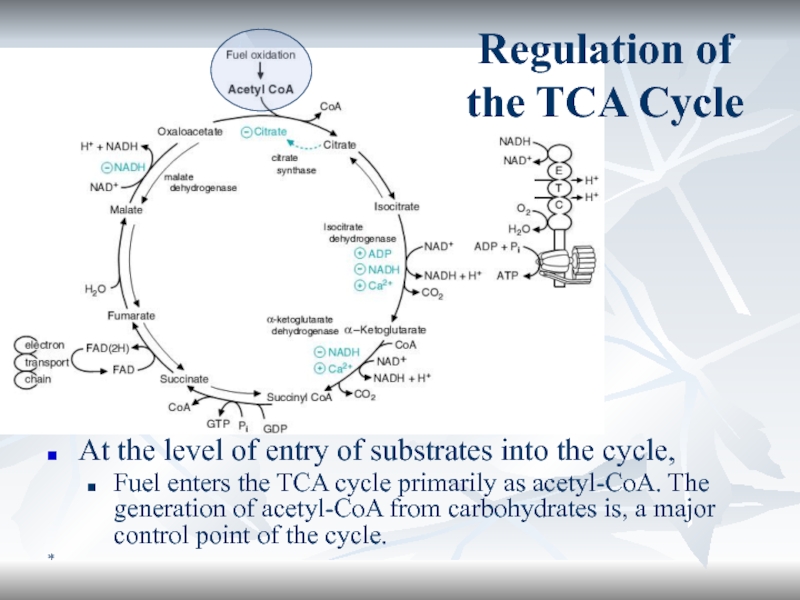
At the level of entry of substrates into the cycle,
Fuel
enters the TCA cycle primarily as acetyl-CoA. The generation of
acetyl-CoA from carbohydrates is, a major control point of the cycle.Regulation of the TCA Cycle
Слайд 35*
Regulation of the TCA Cycle (cont’d)
At the key reactions of
the cycle.
3 reactions of the TCA cycle utilize NAD+
as cofactor ? the cellular ratio of NAD+/NADH has a major impact on the flux of carbon through the TCA cycle. Substrate availability. Citrate synthase reaction depends on availability of oxaloacetate.
Product inhibition also controls the TCA flux, e.g. citrate inhibits citrate synthase, α-KGDH is inhibited by NADH and succinyl-CoA.
The key enzymes of the TCA cycle are also regulated allosterically by Ca2+, ATP and ADP.
Слайд 372
Tissue respiration.
Oxidative phosphorylation.
Microsomal oxidation and peroxidation
Alexander KOVAL
PhD,
senior lecturer
Слайд 38*
Content
The Ways of Oxygen Consumption in the Organism
Structure & Functions
of Respiratory Chain
Oxidative Phosphorylation
Microsomal Oxidation. Peroxydase Pathway. Monooxygenase Systems. Dioxygenase
SystemFree Radicals, Peroxidation and Antioxidants
Слайд 40*
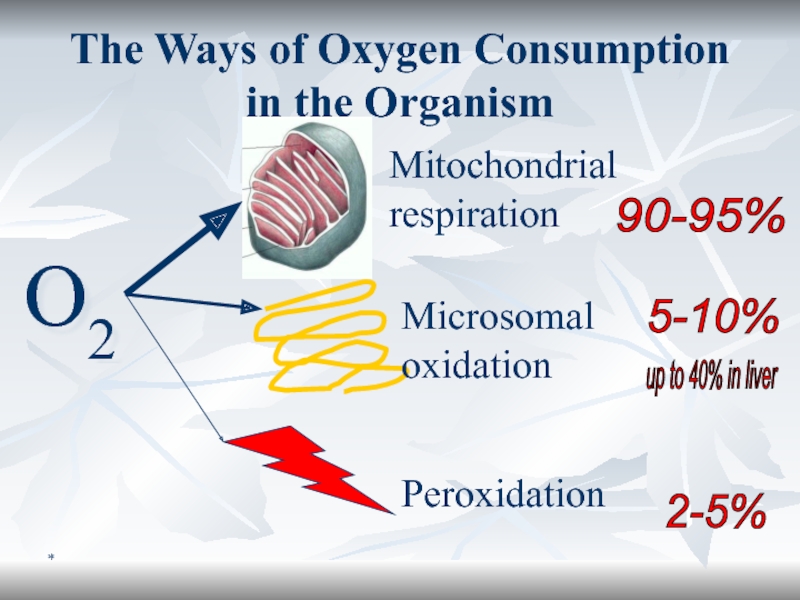
The Ways of Oxygen Consumption in the Organism
O2
Mitochondrial respiration
Microsomal oxidation
Peroxidation
90-95%
5-10%
2-5%
up to 40% in liver
Слайд 41*
Biologic Oxidation (BO)
Oxidation is the removal of electrons and reduction
– the gain of electrons.
Biologic oxidation can take place
without molecular oxygen. Respiration is the process by which cells gain energy in the form of ATP from the controlled reaction of hydrogen with oxygen to form water.
Tissue respiration.
Слайд 42*
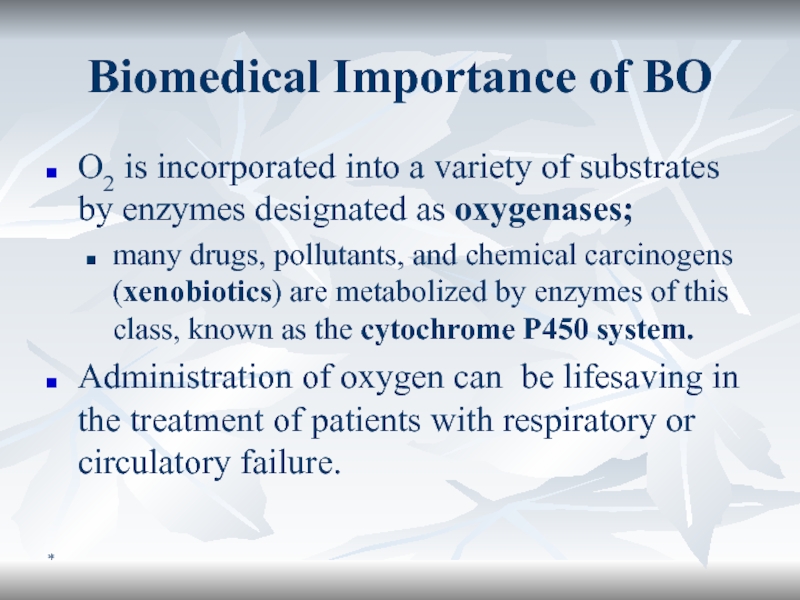
Biomedical Importance of BO
O2 is incorporated into a variety of
substrates by enzymes designated as oxygenases;
many drugs, pollutants, and
chemical carcinogens (xenobiotics) are metabolized by enzymes of this class, known as the cytochrome P450 system. Administration of oxygen can be lifesaving in the treatment of patients with respiratory or circulatory failure.
Слайд 43*
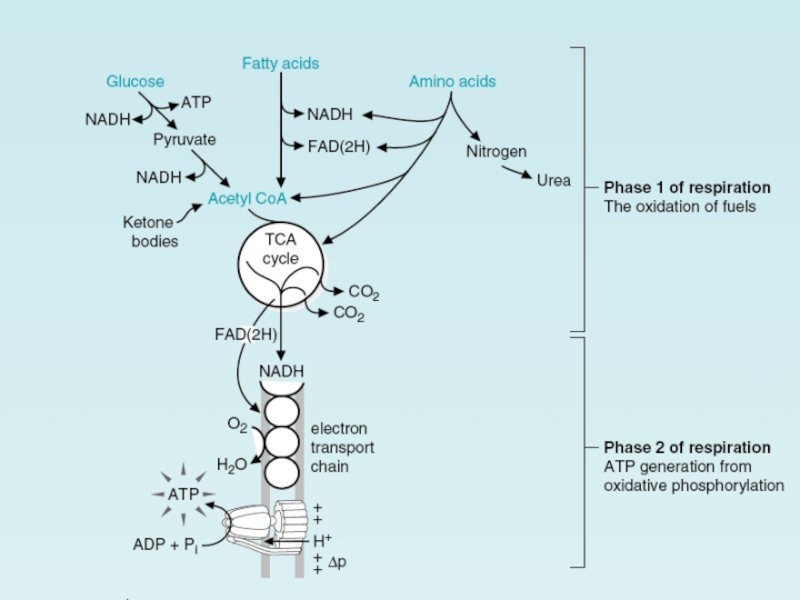
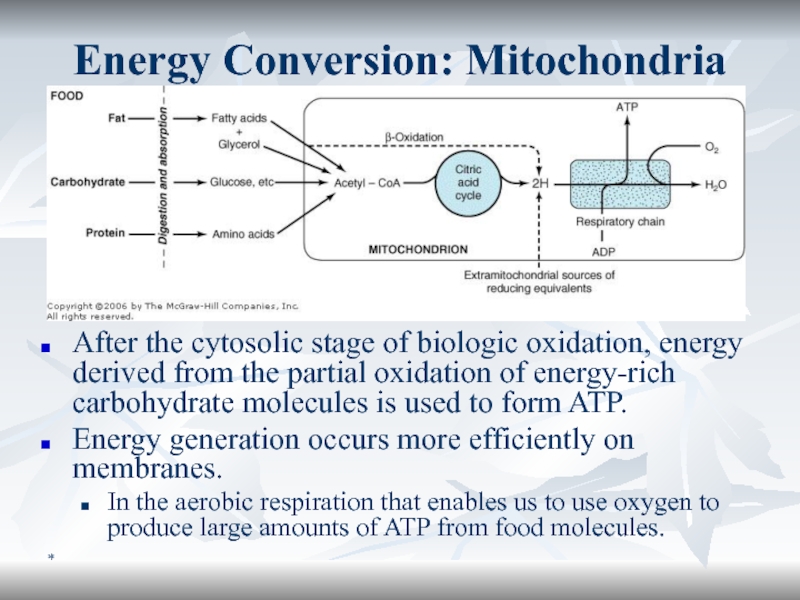
Energy Conversion: Mitochondria
After the cytosolic stage of biologic oxidation, energy
derived from the partial oxidation of energy-rich carbohydrate molecules is
used to form ATP.Energy generation occurs more efficiently on membranes.
In the aerobic respiration that enables us to use oxygen to produce large amounts of ATP from food molecules.
Слайд 44*
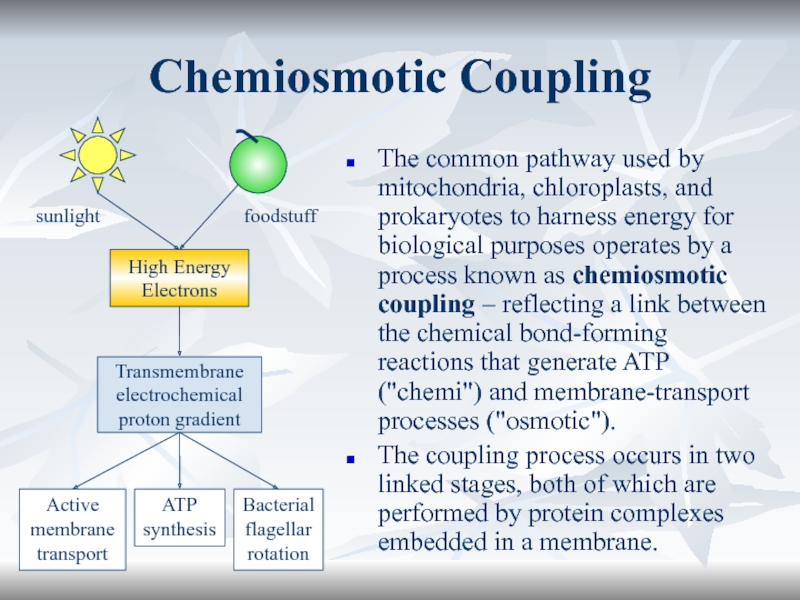
Chemiosmotic Coupling
The common pathway used by mitochondria, chloroplasts, and prokaryotes
to harness energy for biological purposes operates by a process
known as chemiosmotic coupling – reflecting a link between the chemical bond-forming reactions that generate ATP ("chemi") and membrane-transport processes ("osmotic").The coupling process occurs in two linked stages, both of which are performed by protein complexes embedded in a membrane.
sunlight
foodstuff
High Energy
Electrons
Transmembrane
electrochemical
proton gradient
Active
membrane
transport
ATP
synthesis
Bacterial
flagellar
rotation
Слайд 45*
Electron Transporting Chain, ETC (1/2)
The mitochondria contain the series of
catalysts known as the respiratory chain (electron transporting chain, ETC)
that collect and transport reducing equivalents and direct them to their final reaction with oxygen to form water.ETC components are imbedded to the inner membrane of mitochondria.
Слайд 47*
ETC functions
It is the final common pathway in aerobic cells.
NAD+
and FAD are reduced to NADH + H+ and FADH2
respectively in most oxidation reactions. By ETC these coenzymes are reoxidized to NAD+ and FAD.Слайд 49*
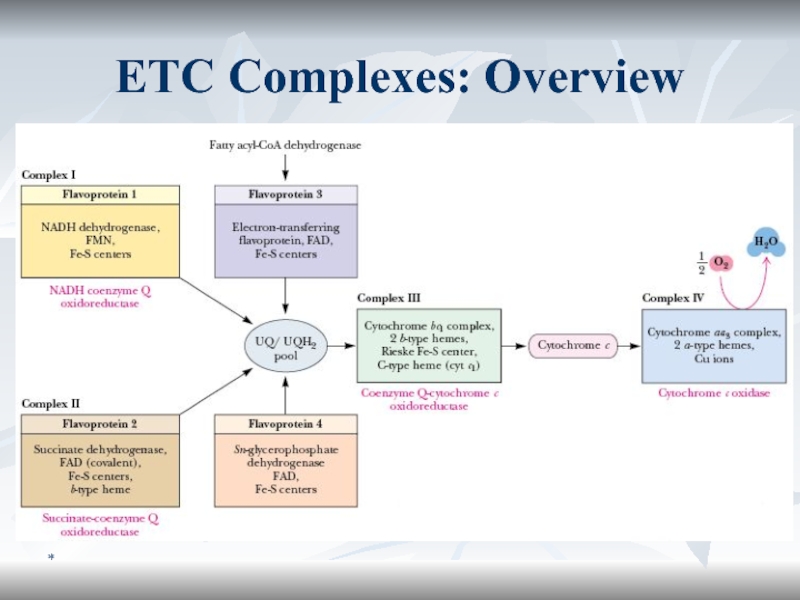
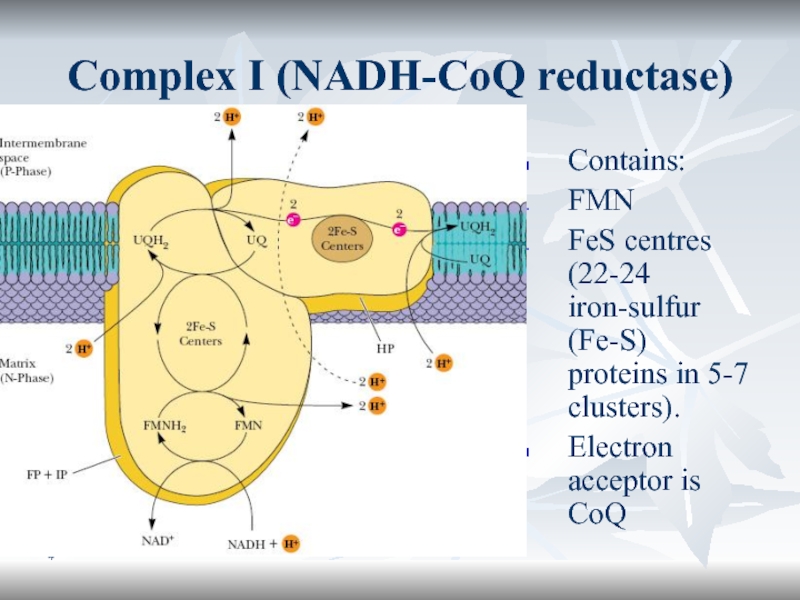
Complex I (NADH-CoQ reductase)
Contains:
FMN
FeS centres (22-24 iron-sulfur (Fe-S) proteins in
5-7 clusters).
Electron acceptor is CoQ
Слайд 50*
Coenzyme Q (CoQ) or Ubiquinone
CoQ is a component of the
inner mitochondrial membrane involved in the process of electron transport.
It draws electrons into the respiratory chain, not only from NADH but also from succinate.
Слайд 51*
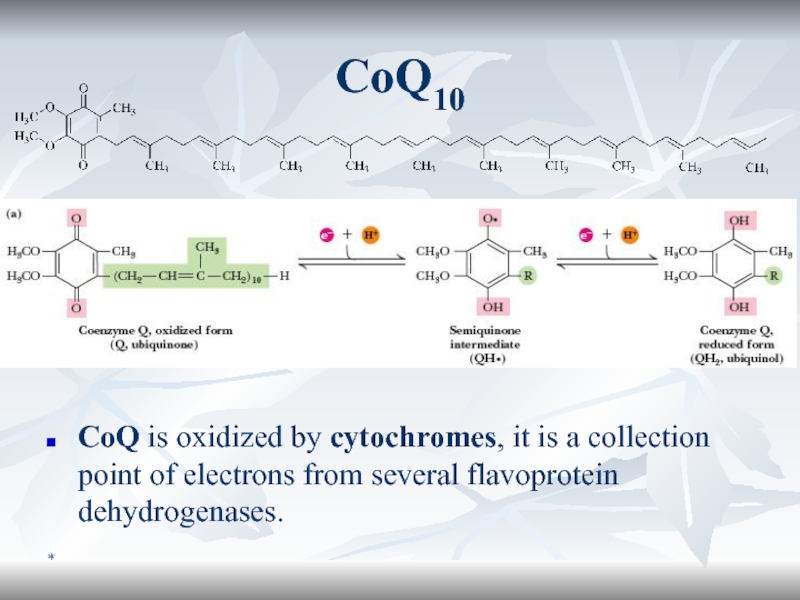
CoQ10
CoQ is oxidized by cytochromes, it is a collection point
of electrons from several flavoprotein dehydrogenases.
Слайд 52*
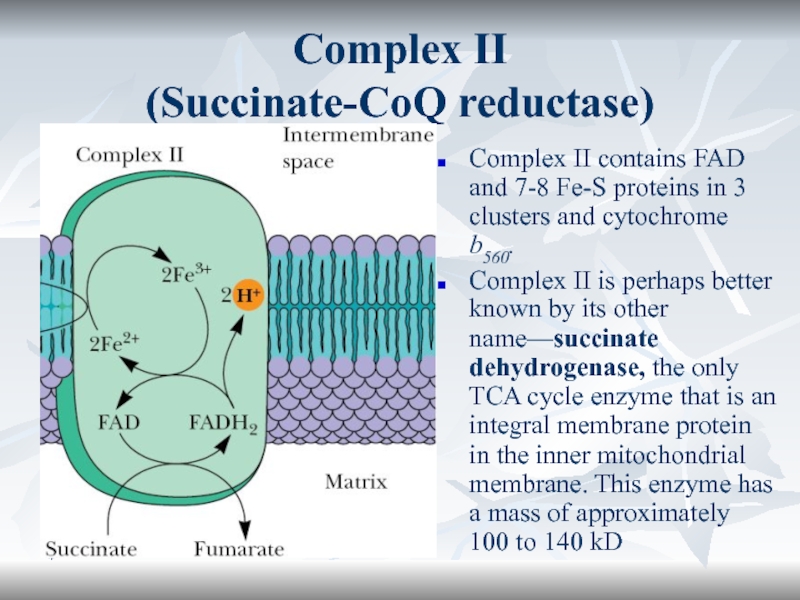
Complex II
(Succinate-CoQ reductase)
Complex II contains FAD and 7-8 Fe-S
proteins in 3 clusters and cytochrome b560.
Complex II is perhaps
better known by its other name—succinate dehydrogenase, the only TCA cycle enzyme that is an integral membrane protein in the inner mitochondrial membrane. This enzyme has a mass of approximately 100 to 140 kD Слайд 53*
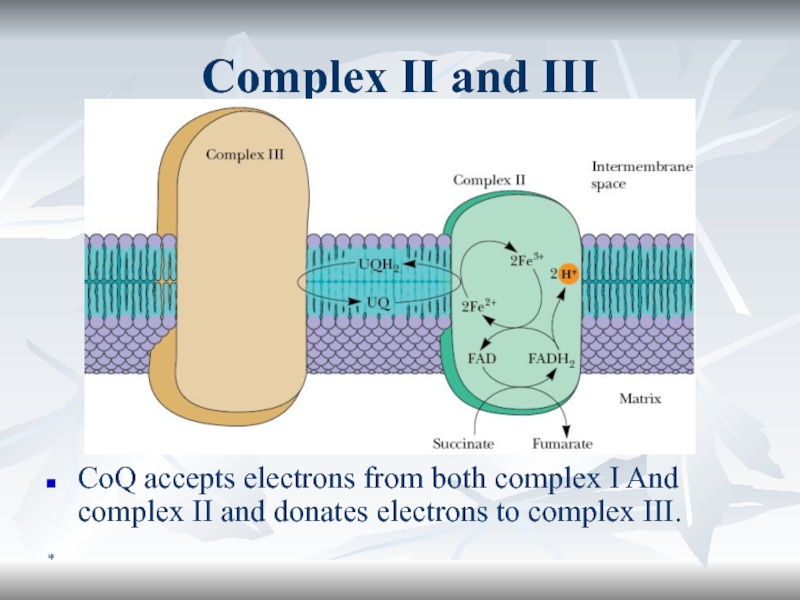
Complex II and III
CoQ accepts electrons from both complex I
And complex II and donates electrons to complex III.
Слайд 54*
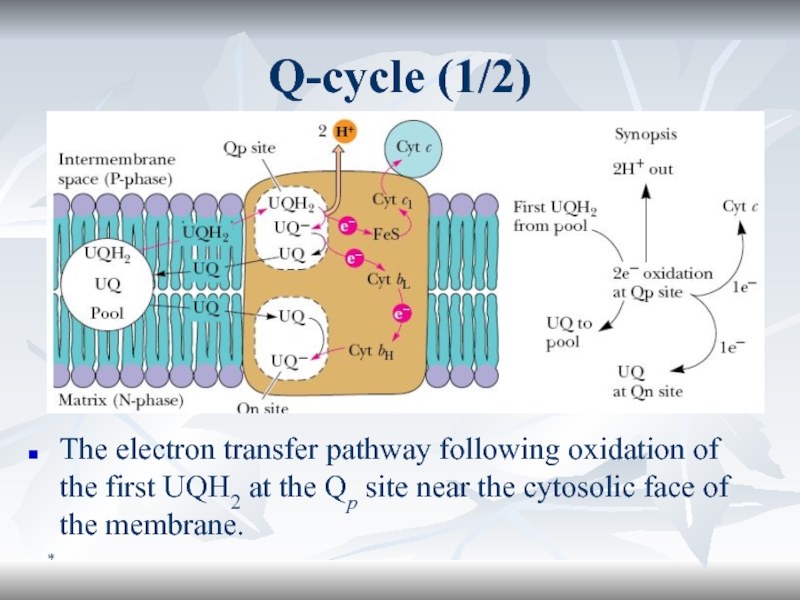
Q-cycle (1/2)
The electron transfer pathway following oxidation of the first
UQH2 at the Qp site near the cytosolic face of
the membrane.Слайд 56*
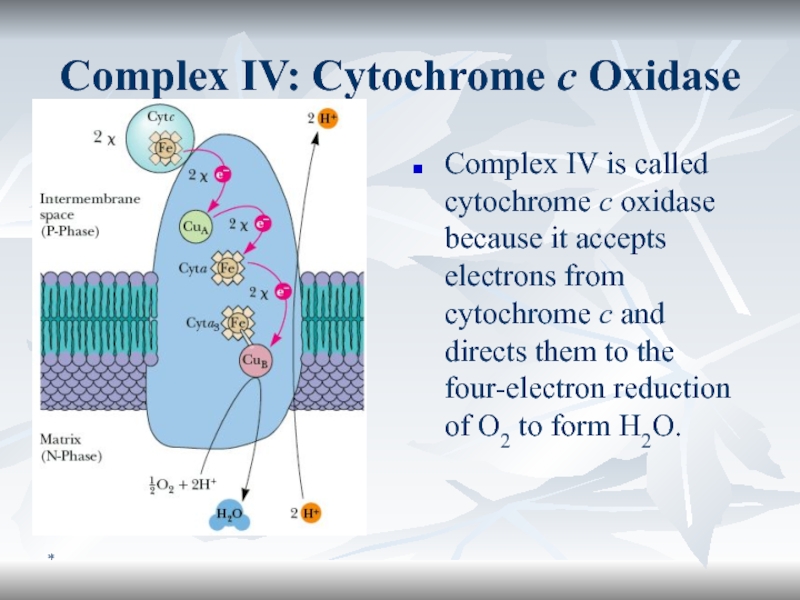
Complex IV: Cytochrome c Oxidase
Complex IV is called cytochrome c
oxidase because it accepts electrons from cytochrome c and directs
them to the four-electron reduction of O2 to form H2O.Слайд 57*
H+-ATPase
Ion gradient across a membrane is a form of stored
energy, which can do useful work when the ions are
flowing back across the membrane.H+ flows back down its electrochemical gradient through ATP synthase, which catalyzes the synthesis of ATP from ADP and inorganic phosphate (Pi).
This ubiquitous enzyme plays the role of a turbine, permitting the proton gradient to drive the production of ATP.
Слайд 58*
ATP/ADP translocase
Outward transport of ATP (via the ATP/ADP translocase) is
favored by the membrane electrochemical potential.
Слайд 60*
Functional scheme of ETC
There are 3 cycles in ETC functioning:
F-cycle, Q-cycle and O-cycle.
Proton pumping result in electrochemical gradient
ΔμH+ formation. Finally it is used for ATP formation.
4H+
4H+
2H+
4H+
2H+
2H+
NADH + H+
NAD+
FADH2
FAD
TCA
Q-cycle
O-cycle
4H+ + O2
H2O
ADP + Pi
ATP
H+
ATP
synthase
QH2
F-cycle
Слайд 65*
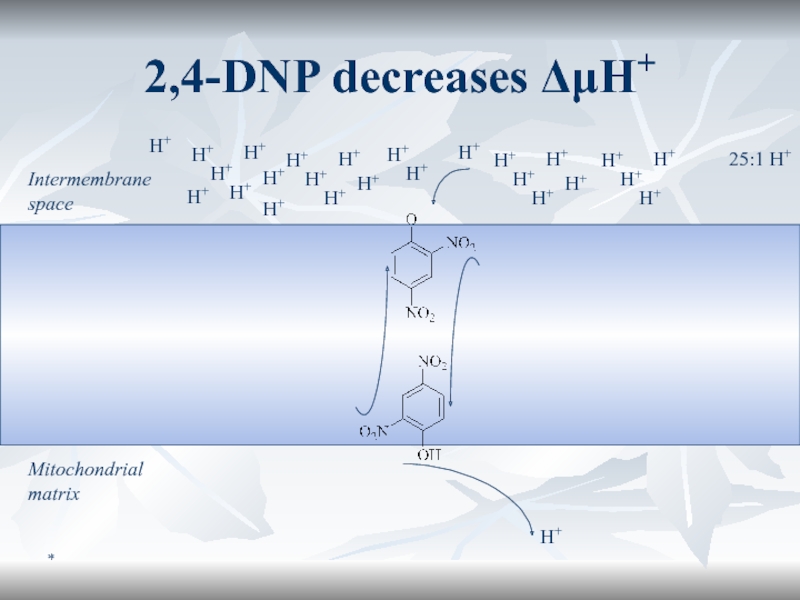
Uncoupler Action
2,4-Dinitrophenol (2,4-DNP) can uptake proton from the intermembrane space
and transports it back to the mitochondrial matrix.
Слайд 66*
2,4-DNP decreases ΔμH+
Intermembrane space
Mitochondrial matrix
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
25:1 H+
Слайд 67*
Endogenous Uncouplers Enable Organisms To Generate Heat
Certain cold-adapted animals, hibernating
animals, and newborn animals generate large amounts of heat by
uncoupling oxidative phosphorylation.Adipose tissue in these organisms contains so many mitochondria that it is called brown adipose tissue for the color imparted by the mitochondria.
The inner membrane of brown adipose tissue mitochondria contains an endogenous protein called thermogenin (literally, “heat maker”), or uncoupling protein, that creates a passive proton channel through which protons flow from the cytosol to the matrix.
Слайд 68*
P/O Ratio
Electrons that enter the chain from NADH supports the
synthesis of ≈3 moles of ATP.
Electrons that enter the chain
from FADH2 supports the synthesis of ≈2 moles of ATP.The P/O ratio refers to the number of inorganic phosphate molecules utilized for ATP generation for every atom of oxygen consumed.
NADH P/O = 3
FADH2 P/O = 2
Ascorbate P/O = 1
Слайд 69*
Disorders of Mitochondrial Oxidative Phosphorylation
Mitochondria contain DNA (mtDNA).
Some components of
ETC are coded in mtDNA. Others – in nuclear DNA.
Several
disorders of OP are the result of mtDNA damage. Слайд 70*
Clinical Manifestation and Treatment of Mito Disorders
Manifestation
Muscle cramping and weakness,
Fatigue,
Lactic
acidosis,
CNS dysfunction,
Vision problems.
Treatment
Is difficult and often unsuccesfull
In some cases
can be helpful ubiquinone, vitamin C, menadione.Слайд 71*
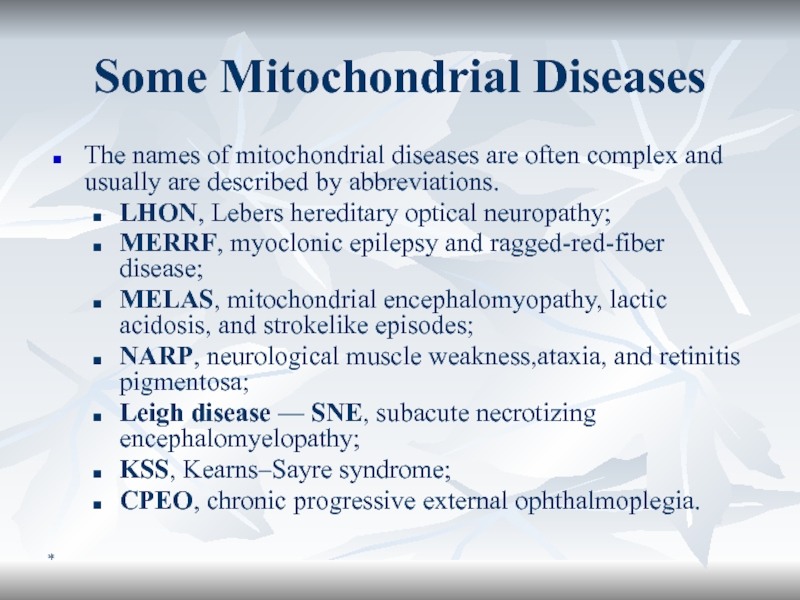
Some Mitochondrial Diseases
The names of mitochondrial diseases are often complex
and usually are described by abbreviations.
LHON, Lebers hereditary optical
neuropathy; MERRF, myoclonic epilepsy and ragged-red-fiber disease;
MELAS, mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes;
NARP, neurological muscle weakness,ataxia, and retinitis pigmentosa;
Leigh disease — SNE, subacute necrotizing encephalomyelopathy;
KSS, Kearns–Sayre syndrome;
CPEO, chronic progressive external ophthalmoplegia.
Слайд 72*
LHON
LHON is a hereditary disease that often leads to sudden
blindness from death of the optic nerve especially among males.
Any one of several point mutations in
subunits ND1, 2, 4, 5, and 6 of NADH dehydrogenase (complex I),
cytochrome b of complex II, or
subunit I of cytochrome oxidase
may cause this syndrome.
Most frequent is an R340H mutation of the ND4 gene at position 11,778 of mtDNA. It may interfere with reduction of ubiquinone.
Mutations in the ND1 gene at position 3460 and in the ND6 gene at position 14484 or in the cytochrome b gene at position 15257 cause the same disease.
Слайд 73*
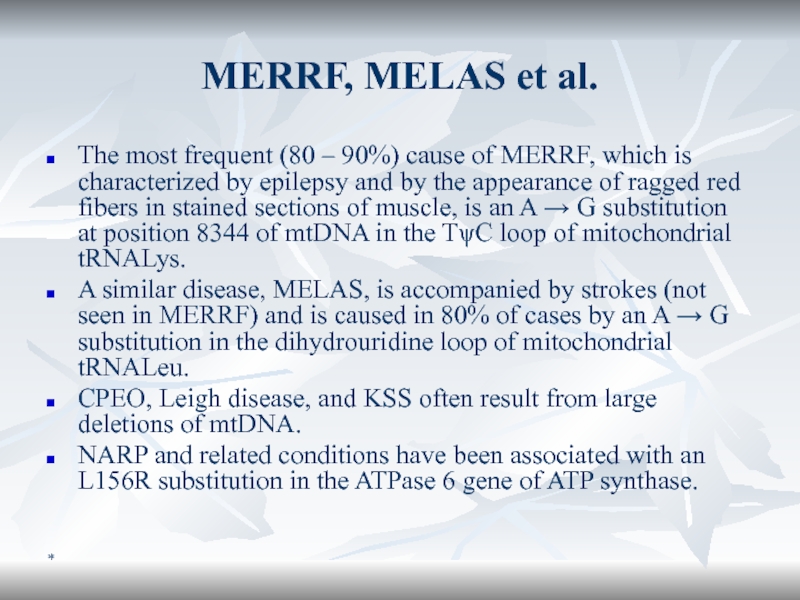
MERRF, MELAS et al.
The most frequent (80 – 90%) cause
of MERRF, which is characterized by epilepsy and by the
appearance of ragged red fibers in stained sections of muscle, is an A → G substitution at position 8344 of mtDNA in the TψC loop of mitochondrial tRNALys.A similar disease, MELAS, is accompanied by strokes (not seen in MERRF) and is caused in 80% of cases by an A → G substitution in the dihydrouridine loop of mitochondrial tRNALeu.
CPEO, Leigh disease, and KSS often result from large deletions of mtDNA.
NARP and related conditions have been associated with an L156R substitution in the ATPase 6 gene of ATP synthase.
Слайд 74*
Can Mitochondrial Diseases be Treated?
Attempts are being made to
improve the function of impaired mitochondria by adding large amounts
of ubiquinone, vitamin K, thiamin, riboflavin, and succinate to the diet.One report suggests that mitochondrial decay during aging can be reversed by administration of N-acetylcarnitine.
Слайд 75*
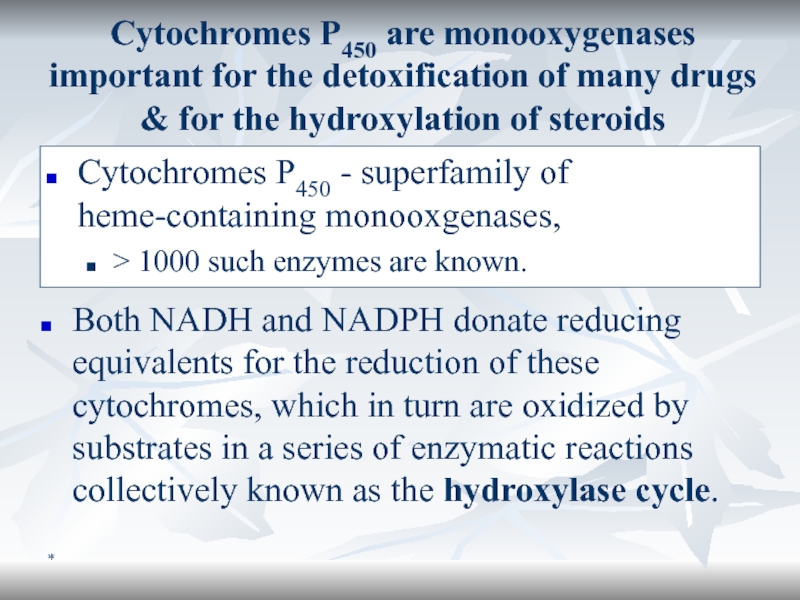
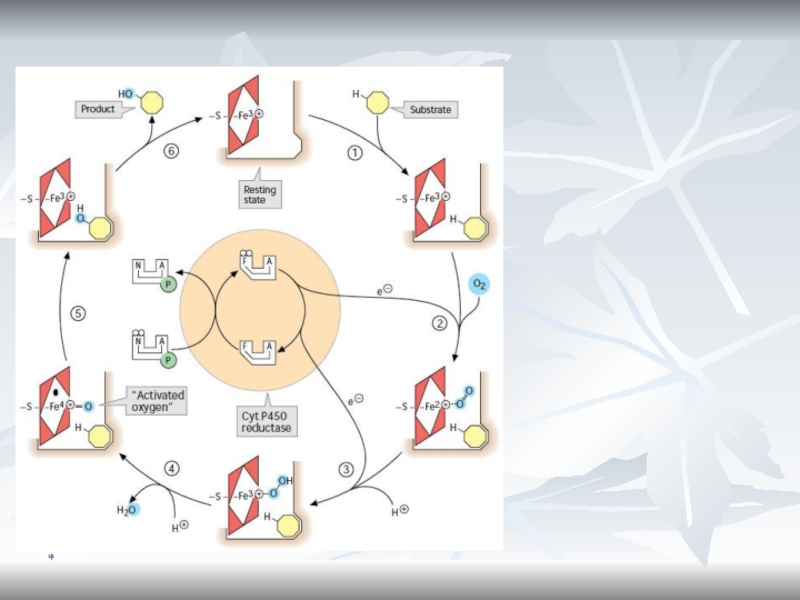
Cytochromes P450 are monooxygenases important for the detoxification of many
drugs & for the hydroxylation of steroids
Cytochromes P450 - superfamily
of heme-containing monooxgenases, > 1000 such enzymes are known.
Both NADH and NADPH donate reducing equivalents for the reduction of these cytochromes, which in turn are oxidized by substrates in a series of enzymatic reactions collectively known as the hydroxylase cycle.
Слайд 76*
Cytochrome b5
In liver microsomes, cytochromes P450 are found together with
cytochrome b5 and have an important role in detoxification.
Benzpyrene,
aminopyrine, aniline, morphine, and benzphetamine are hydroxylated, increasing their solubility and aiding their excretion. Many drugs such as phenobarbital have the ability to induce the formation of microsomal enzymes and of cytochromes P450.
Слайд 77*
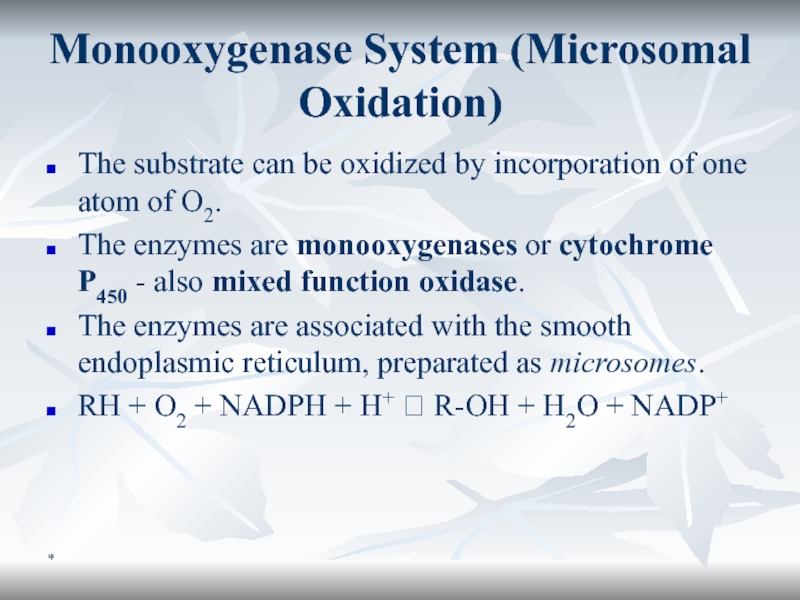
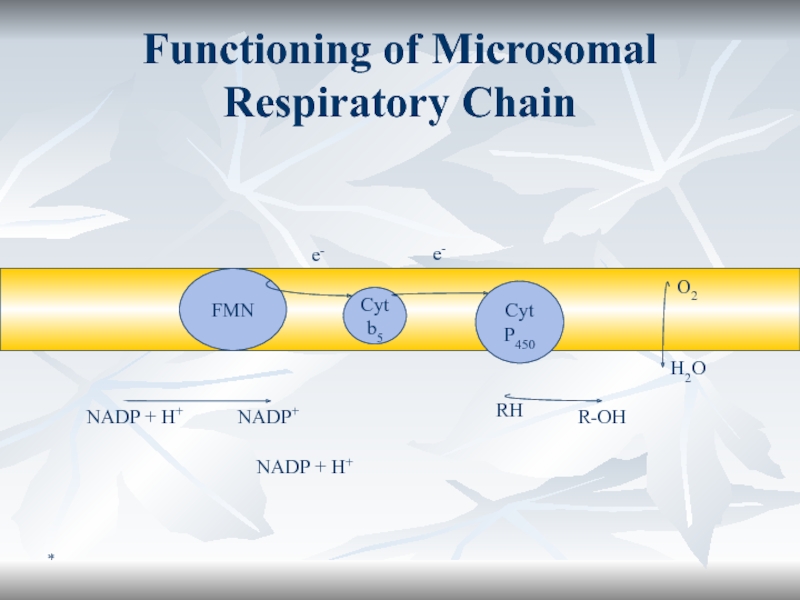
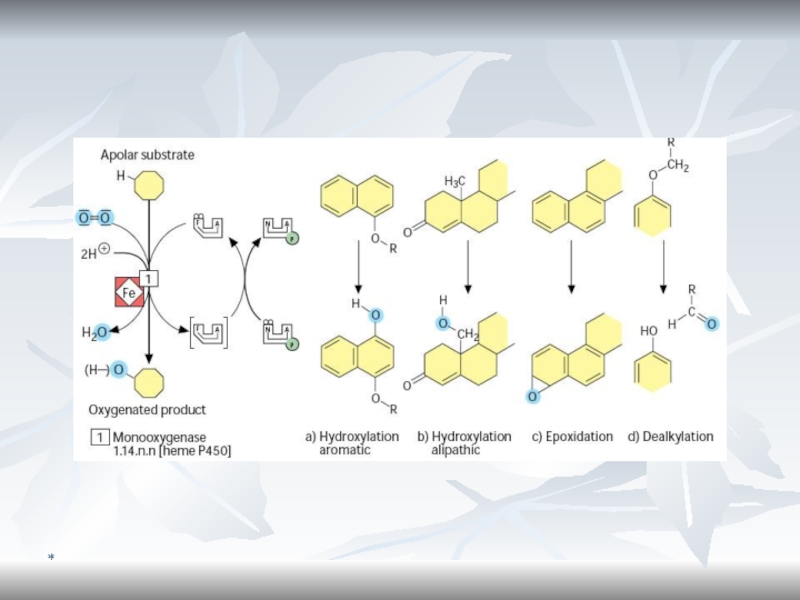
Monooxygenase System (Microsomal Oxidation)
The substrate can be oxidized by incorporation
of one atom of O2.
The enzymes are monooxygenases or
cytochrome P450 - also mixed function oxidase. The enzymes are associated with the smooth endoplasmic reticulum, preparated as microsomes.
RH + O2 + NADPH + H+ ? R-OH + H2O + NADP+









![Биологическое окисление *Maкроэргичность ATФОтрицательно заряженный «хвост» создает сильное отталкиваниеATФ4- ? ADФ3- + Фн2- *Maкроэргичность ATФОтрицательно заряженный «хвост» создает сильное отталкиваниеATФ4- ? ADФ3- + Фн2- + H+[ATФ4-] = [ADФ3-] = []](/img/thumbs/cf0527dd9ee4ddc926cc358177f834e4-800x.jpg)