Слайд 1Pharmacology. lecture 1
Associate-professor Goldobina Galina
Слайд 2Medical pharmacology is the science of chemicals (drugs) that interact
with the human body. These interactions are divided into two
classes:
Pharmacodynamics denotes the actions of the drug on the body, such as mechanism of action and therapeutic and toxic effects.
Pharmacokinetics describes the effects of the body on drugs, eg, absorption, distribution, metabolism and excretion.
Слайд 3Routes of administration: ADVANTAGES and DISADVANTAGES
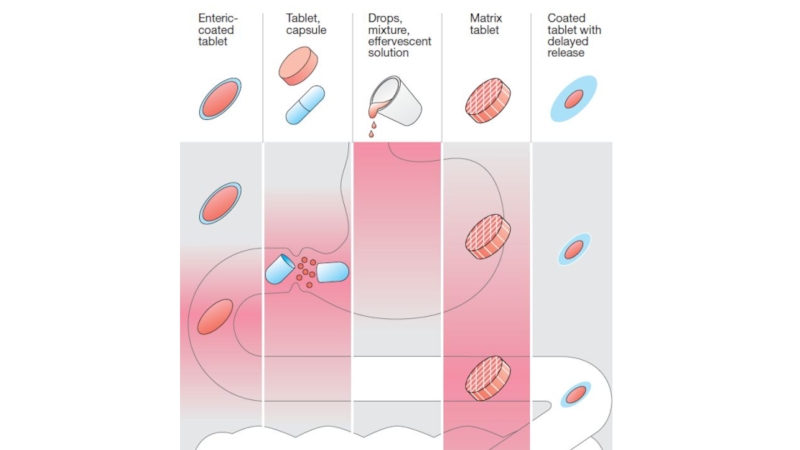
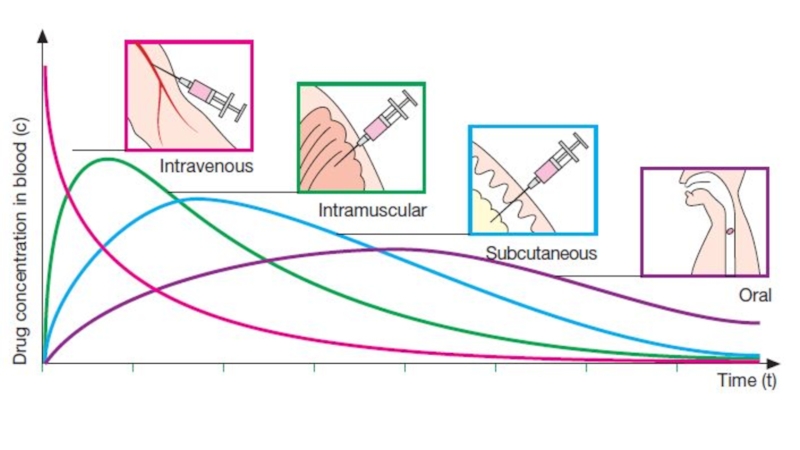
Oral: the most common and
safest, convenient, and economical
route of administration, but absorption
is affected by many factors (food, HCl), drugs may be metabolized before systemic absorption (first-pass effect). Patient compliance is necessary
Sublingual: bypasses first-pass effect, destruction by stomach acid, may cause immediate pharmacological effects, but limited to certain types of drugs, to drugs that can be taken in small doses, may loose part of the drug dose if it is swallowed
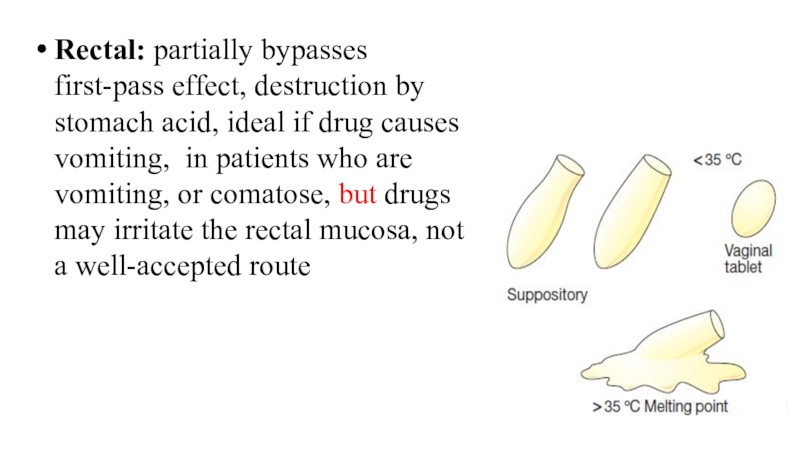
Слайд 5Rectal: partially bypasses first-pass effect, destruction by stomach acid, ideal
if drug causes vomiting, in patients who are vomiting, or
comatose, but drugs may irritate the rectal mucosa, not a well-accepted route
Слайд 6Intravenous: can have immediate effects, ideal if dosed in large
volumes, suitable for irritating substances and complex mixtures, valuable in
emergency situations, dosage titration permissible, ideal for high molecular weight proteins and peptide drugs, but unsuitable for oily substances, may result in adverse effects, most substances must be slowly injected, strict aseptic techniques are needed
Слайд 7Subcutaneous: suitable for slow-release drugs, ideal for some poorly soluble
suspensions, but pain or necrosis if drug is irritating, unsuitable
for drugs administered in large volumes
Intramuscular: suitable if drug volume is moderate, suitable for oily solutions and certain irritating substances, preferable to intravenous if patient must self-administer, but can be painful, can cause intramuscular hemorrhage (precluded during anticoagulation therapy)
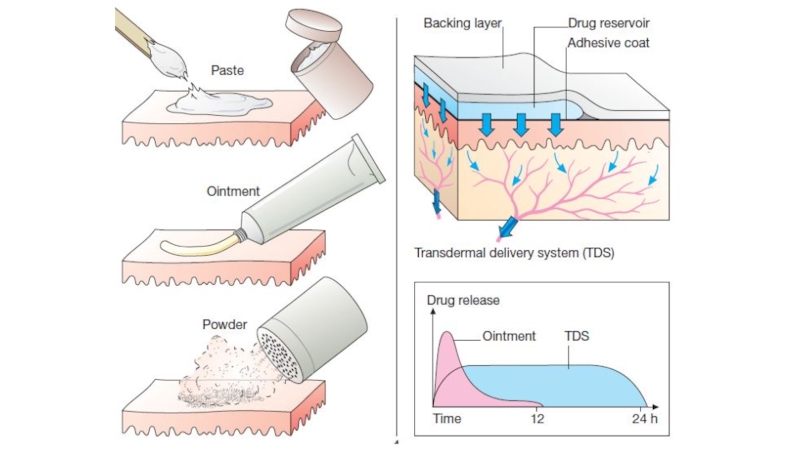
Слайд 9Transdermal (patch): bypasses the first-pass effect, convenient and painless,
ideal for drugs that are lipophilic and have poor oral
bioavailability,
ideal for drugs that are quickly eliminated from the body,
but some patients are allergic to patches, which can cause irritation,
drug must be highly lipophilic, may cause delayed delivery of drug to pharmacological site of action,
limited to drugs that can be taken in small daily doses.
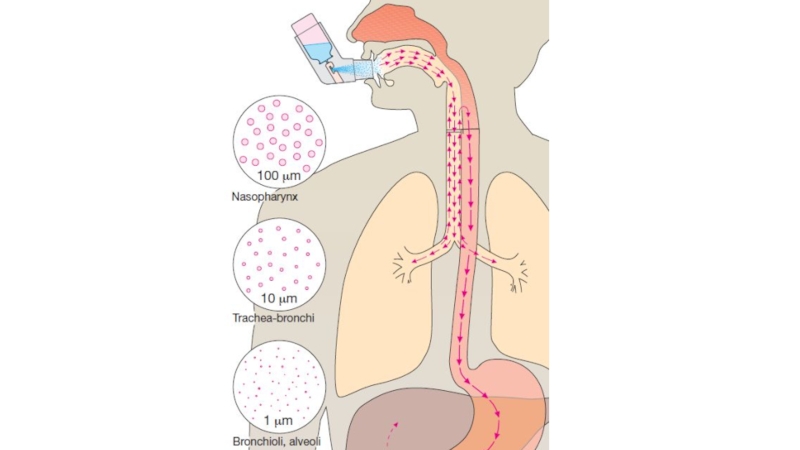
Слайд 11Inhalation: absorption is rapid; can have immediate effects, ideal for
gases,
effective for patients with respiratory problems, dose can
be titrated, localized effect to target lungs: lower doses are used compared to that with oral or parenteral administration, fewer systemic side effects,
But drug can enter the brain quickly, patient may have difficulty regulating dose, some patients may have difficulty using inhalers.
Слайд 13Mechanisms of absorption of drugs
Drugs may be absorbed from the
GI tract by passive diffusion, filtration, facilitated diffusion, active transport,
or pinocytosis.
The driving force for passive absorption (filtration, passive diffusion) of a drug is the concentration gradient across a membrane separating two body compartments. The drug moves from a region of high concentration to one of lower concentration.
Filtration: water-soluble drugs penetrate the cell membrane through aqueous channels or pores.
Слайд 14Diffusion: lipid-soluble drugs readily move across most biologic membranes due
to their solubility in the membrane lipid bilayers. Passive diffusion
does not involve a carrier, is not saturable, and shows a low structural specificity. The vast majority of drugs are absorbed by this mechanism.
Facilitated diffusion: agents can enter the cell through specialized transmembrane carrier proteins, moving them from an area of high concentration to an area of low concentration. It does not require energy, can be saturated, and may be inhibited by compounds that compete for the carrier.
Слайд 15Active transport: Energy-dependent, involves specific carrier proteins. It is capable
of moving drugs against a concentration gradient. The process is
saturable. Active transport systems are selective and may be competitively inhibited by other cotransported substances.
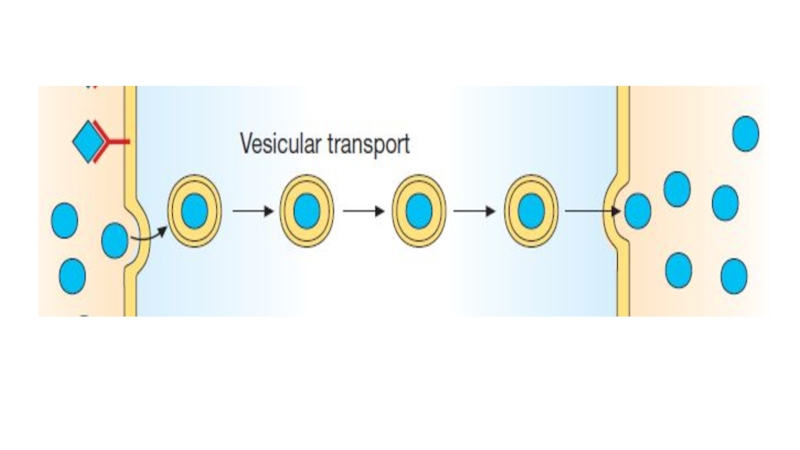
Pinocitosis: This type of absorption is used to transport drugs of exceptionally large size across the cell membrane. It involves engulfment of a drug by the cell membrane and transport into the cell by pinching off the drugfilled vesicle.
Слайд 18Bioavailability
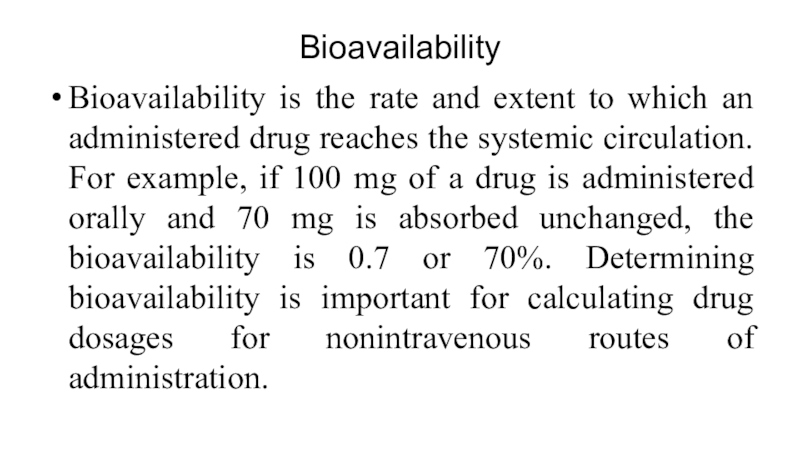
Bioavailability is the rate and extent to which an administered
drug reaches the systemic circulation. For example, if 100 mg
of a drug is administered orally and 70 mg is absorbed unchanged, the bioavailability is 0.7 or 70%. Determining bioavailability is important for calculating drug dosages for nonintravenous routes of administration.
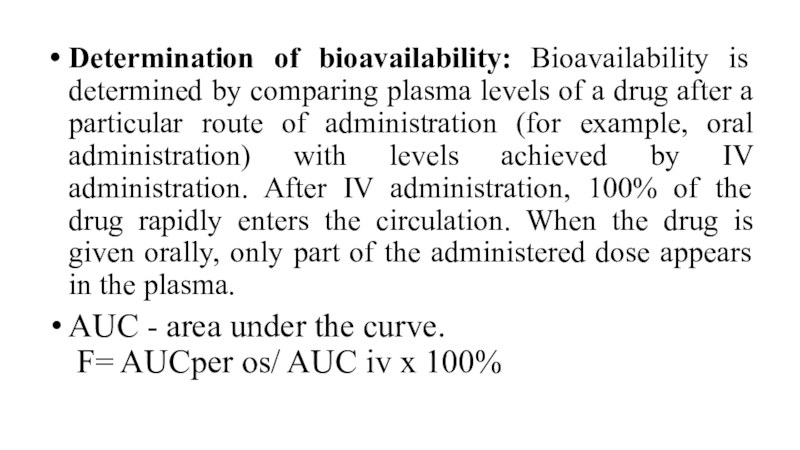
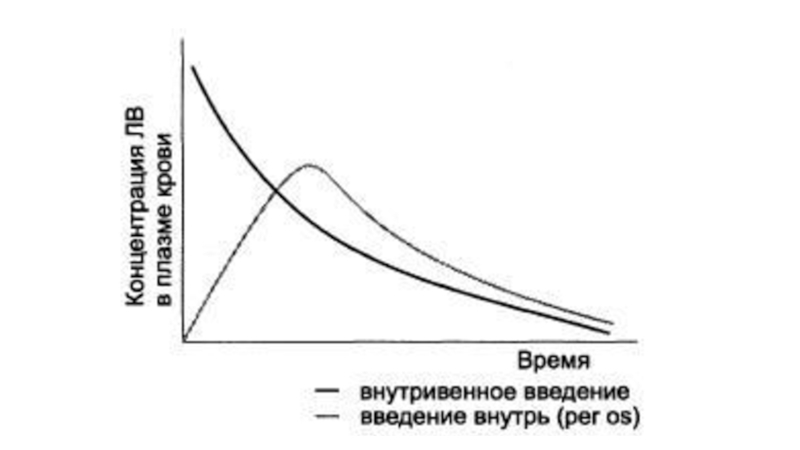
Слайд 19Determination of bioavailability: Bioavailability is determined by comparing plasma levels
of a drug after a particular route of administration (for
example, oral administration) with levels achieved by IV administration. After IV administration, 100% of the drug rapidly enters the circulation. When the drug is given orally, only part of the administered dose appears in the plasma.
AUC - area under the curve.
F= AUCper os/ AUC iv х 100%
Слайд 21Factors that influence bioavailability
First-pass hepatic metabolism: When a drug is
absorbed from the GI tract, it enters the portal circulation
before entering the systemic circulation. If the drug is rapidly metabolized in the liver or gut wall during this initial passage, the amount of unchanged drug entering the systemic circulation is decreased.
Solubility of the drug: For a drug to be readily absorbed, it must be largely lipophilic.
Слайд 22Chemical instability.
Nature of the drug formulation (salt form, crystal polymorphism,
enteric coatings, and the presence of excipients: such as binders
and dispersing agents).
Слайд 23Drug distribution
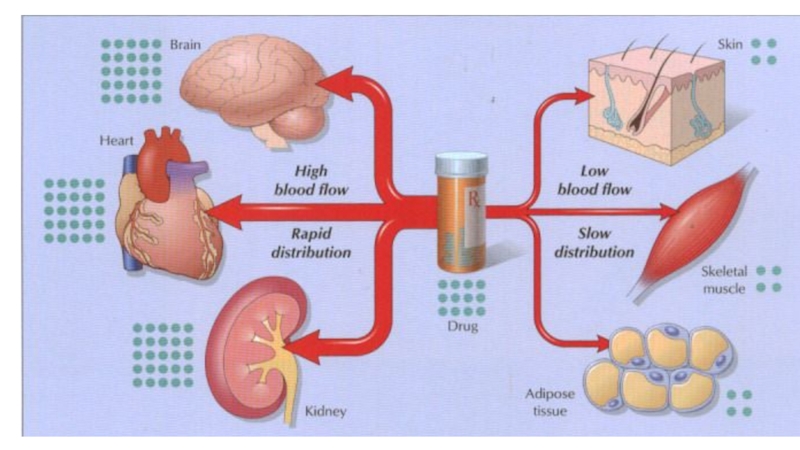
Drug distribution is the process by which a drug
reversibly leaves the bloodstream and enters the interstitium (extracellular fluid)
and the tissues.
The distribution of a drug from the plasma to the interstitium depends on:
cardiac output and local blood flow,
capillary permeability, the tissue volume,
the degree of binding of the drug to plasma and tissue proteins,
and the relative lipophilicity of the drug.
Слайд 24Lipid-soluble drugs readily penetrate the CNS because they dissolve in
the endothelial cell membrane. Ionized or polar drugs generally fail
to enter the CNS because they cannot pass through the endothelial cells that have no slit junctions.
The apparent volume of distribution, Vd, is defined as the fluid volume that is required to contain the entire drug in the body at the same concentration measured in the plasma.
Слайд 27Elimination
Once a drug enters the body, the process of elimination
begins. The three major routes of elimination are hepatic metabolism,
biliary elimination, and urinary elimination.
Renal elimination of a drug via the kidneys into urine involves the processes of glomerular filtration, active tubular secretion, and passive tubular reabsorption.
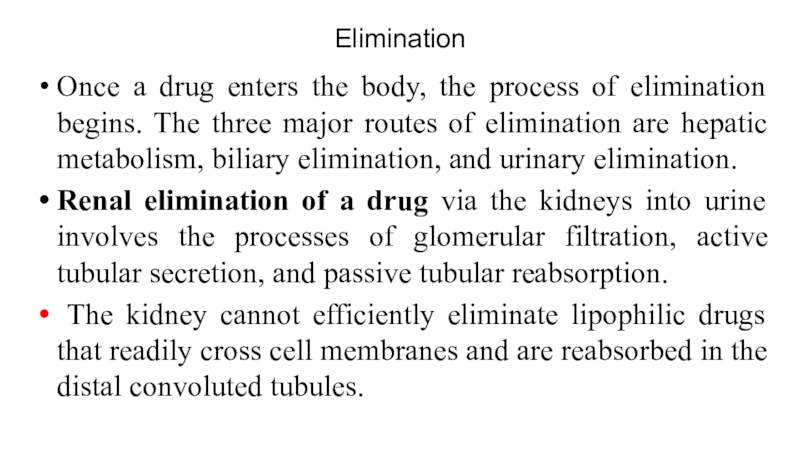
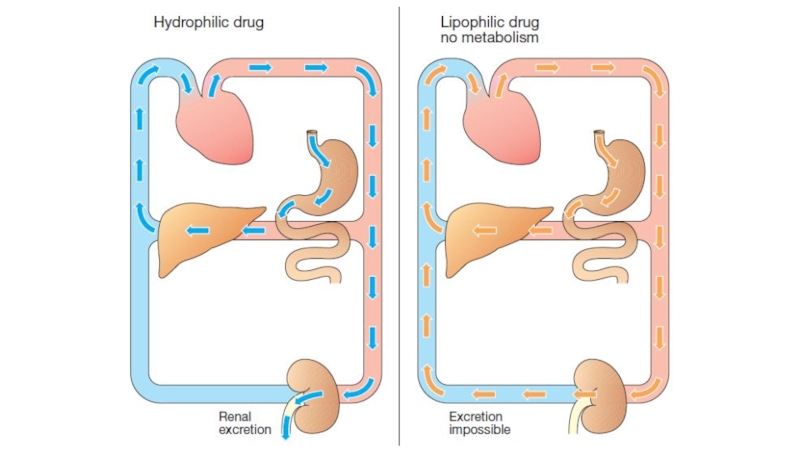
The kidney cannot efficiently eliminate lipophilic drugs that readily cross cell membranes and are reabsorbed in the distal convoluted tubules.
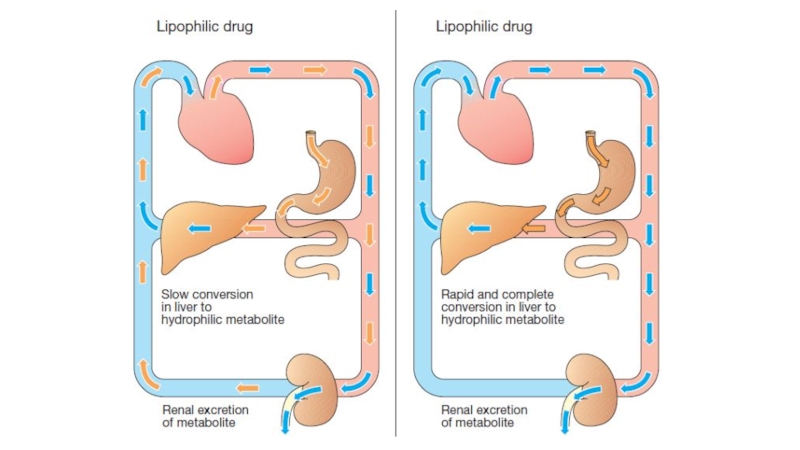
Слайд 29Metabolism in the liver leads to production of products with
increased polarity, which allows the drug to be eliminated.
There are
two general sets of reactions, called phase I and phase II
Phase I: reactions utilizing the P450 system convert lipophilic drugs into more polar molecules. Phase I reactions usually involve reduction, oxidation, or hydrolysis. Phase I metabolism may increase, decrease, or have no effect on pharmacologic activity.
Слайд 31The CYP450-dependent enzymes are an important target for pharmacokinetic drug
interactions.
Inducers (for example, phenobarbital, rifampin, and carbamazepine) are capable
of increasing the synthesis of one or more enzymes.
The more important CYP inhibitors are erythromycin, ketoconazole, because they each inhibit several enzymes.
Слайд 32Phase II: This phase consists of conjugation reactions with an
endogenous substrate, such as glucuronic acid, sulfuric acid, acetic acid,
or an amino acid, results in polar, usually more water-soluble compounds that are often therapeutically inactive. But morphine-6-glucuronide, which is more potent than morphine. Glucuronidation is the most common and the most important conjugation reaction.
The highly polar drug conjugates are then excreted by the kidneys or in bile.
Слайд 33Drug clearance may also occur via the intestines, bile, lungs,
and breast milk, among others. Drugs that are not absorbed
after oral administration or drugs that are secreted directly into the intestines or into bile are eliminated in the feces. The lungs are primarily involved in the elimination of anesthetic gases.
Clearance (CL) estimates the amount of drug cleared from the body per unit of time.
Total CL reflects all mechanisms of drug elimination.
Слайд 34Pharmacodynamics. Effects
Local effect occurs at the site of drug’s
application .
Resorptive (systemic) effect develops after absorption of the drug
into the blood.
Direct effect occurs at the site of contact of the drug with the tissue.
Reflect effect is produced when substances influence extero- or interoceptors causing changes in the status of nerve centres or effector organs.
Слайд 35Drugs “’targets
Few drugs (e.g. activated charcoal, osmotic diuretics) act by
virtue of their physicochemical properties, and this is called non‐specific
drug action.
Some drugs act as false substrates or inhibitors for certain transport systems or enzymes.
Слайд 36Enzymes. Drugs that act by inhibiting enzymes include:
anticholinesterases, which
enhance the action of acetylcholine;
carbonic anhydrase inhibitors, which are
diuretics (i.e. increase urine flow);
monoamine oxidase inhibitors, which are antidepressants;
and inhibitors of cyclooxygenase (NSAIDs) .
Слайд 37Drugs can influence on ion channels (selective pores in the
membrane).
Among drugs affecting ion channels there are local anesthetics,
antiarhythmic, antiepileptic drugs.
Слайд 38However, most drugs produce their effects by acting on specific
proteins. These proteins are called receptors, and they normally respond
to endogenous chemicals in the body. These chemicals are either synaptic transmitter substances or hormones. For example, acetylcholine is a transmitter substance released from motor nerve endings.
Слайд 39A receptor as any biologic molecule to which a drug
binds and produces a measurable response. The receptors may be
divided into four families:
1) ligand-gated ion channels (nicotinic receptor, γ‐aminobutyric acid (GABA) receptor),
2) G protein–coupled receptors,
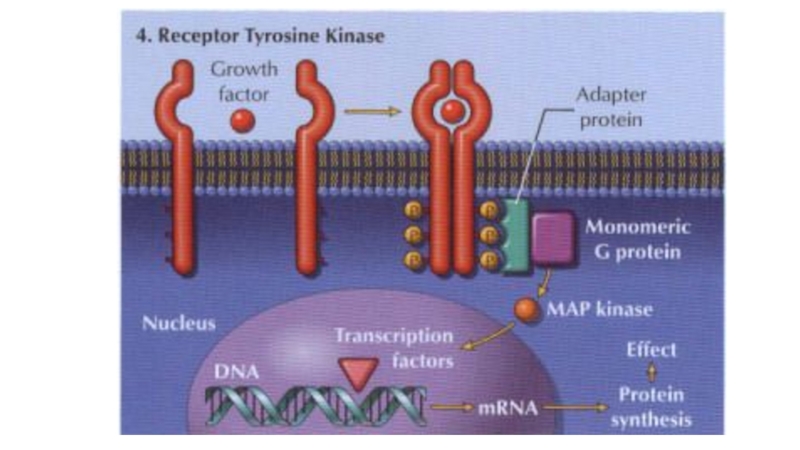
Слайд 423) Kinase‐linked receptors are surface receptors that possess (usually) intrinsic
tyrosine kinase activity. They include receptors for insulin, cytokines and
growth factors.
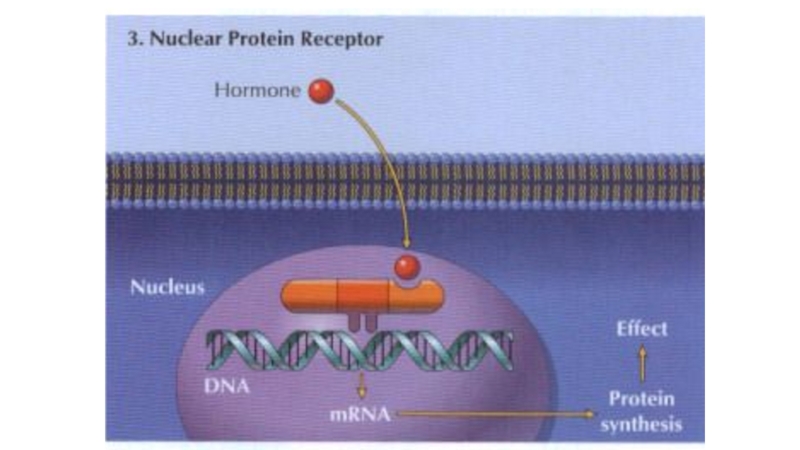
4) Nuclear receptors for steroid hormones and thyroid hormones are present in the cell nucleus and regulate transcription and thus protein synthesis.
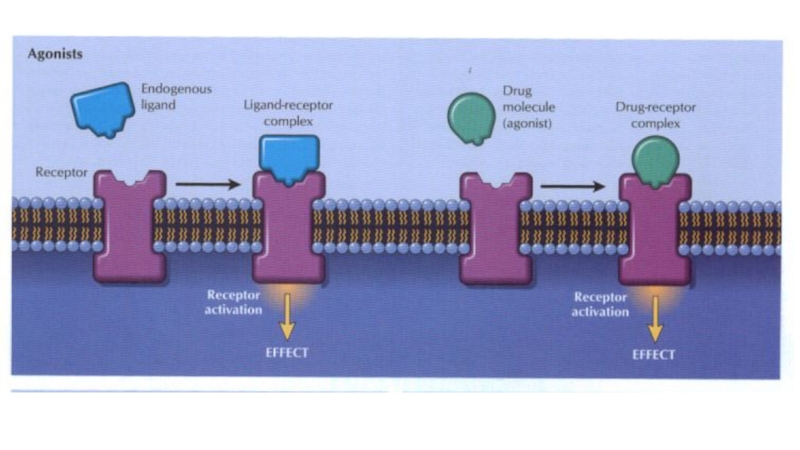
Слайд 45Chemicals (e.g. acetylcholine) or drugs that activate receptors and produce
a response are called agonists .
Affinity is a measure of
how avidly a drug binds to its receptor.
Intrinsic efficacy. This is the ability of an agonist to alter the conformation of a receptor in such a way that it elicits a response in the system.
The interaction between a drug and the binding site of the receptor depends on the complementarity of ‘fit’ of the two molecules.
Слайд 46Partial agonists. These are agonists that cannot elicit the same
maximum response as a ‘full’ agonist.
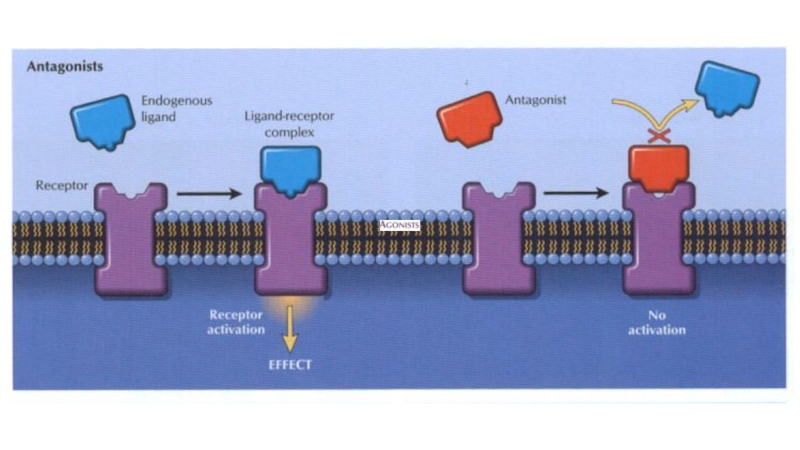
Some drugs, called antagonists,
combine with receptors, but do not activate them. Antagonists reduce the probability of the transmitter substance (or another agonist) combining with the receptor and so reduce or block its action.
Слайд 49The durability of the “Drug-receptor” bond determines whether the drug
action is reversible (characteristic for most drugs) or irreversible (in
case of covalent bond).
The ability of a drug to combine with one particular type of receptor is called specificity. No drug is truly specific, but many have a relatively selective action on one type of receptor.
Drugs are prescribed to produce a therapeutic effect, but they often produce additional unwanted effects or side (adverse effects) that range from the trivial (e.g. slight nausea) to the fatal (e.g. aplastic anaemia).
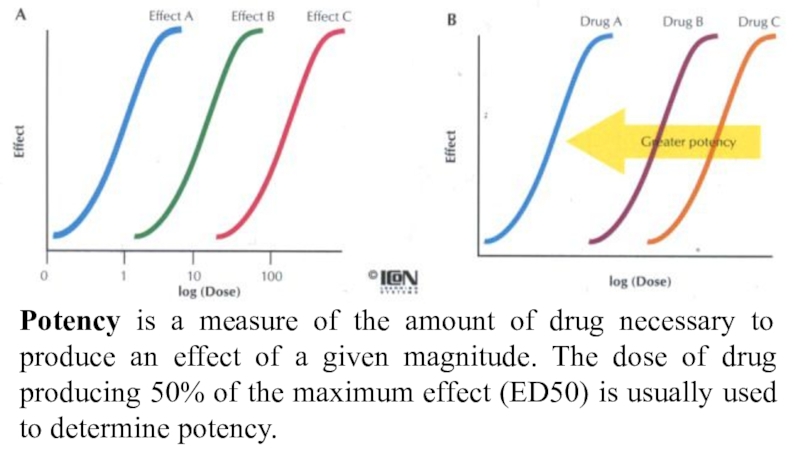
Слайд 50Potency is a measure of the amount of drug necessary
to produce an effect of a given magnitude. The dose
of drug producing 50% of the maximum effect (ED50) is usually used to determine potency.
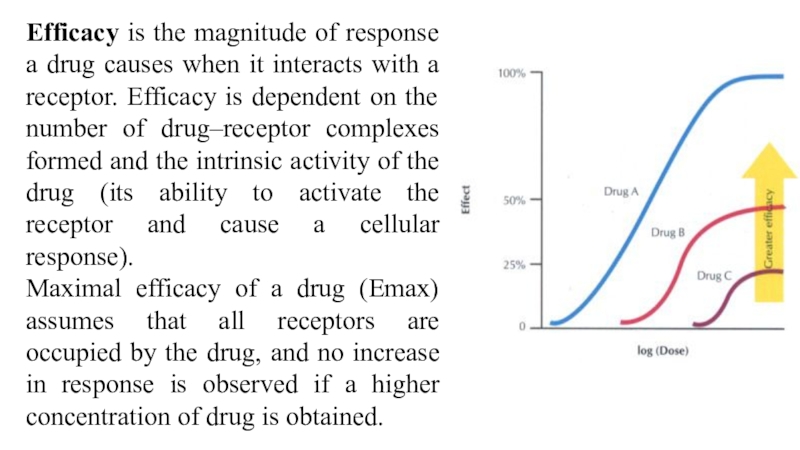
Слайд 51Efficacy is the magnitude of response a drug causes when
it interacts with a receptor. Efficacy is dependent on the
number of drug–receptor complexes formed and the intrinsic activity of the drug (its ability to activate the receptor and cause a cellular response).
Maximal efficacy of a drug (Emax) assumes that all receptors are occupied by the drug, and no increase in response is observed if a higher concentration of drug is obtained.
Слайд 52Drugs interactions
Pharmacokinetic
Pharmacodynamic:
Synergism
Antagonism
Слайд 53In case of synergism drug interaction leads to an increase
in effect. Synergism may be direct (if both compounds affect
one substrate) or indirect (if their effects have different localization).
Diuretics enhance the effect of angiotensin-converting enzyme inhibitors in the treatment of hypertension (indirect).
Paracetamol enhances the effect of metamizole (direct).
Drugs with similar actions, e.g. benzodiazepines and alcohol, produce additive effects and may cause severe central nervous system depression.
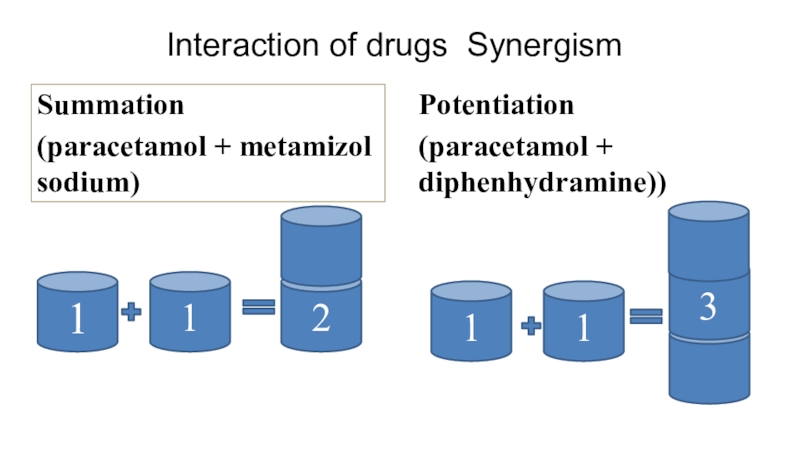
Слайд 54Interaction of drugs Synergism
Summation
(paracetamol + metamizol sodium)
Potentiation
(paracetamol + diphenhydramine))
1
1
2
1
1
3
Слайд 55Antagonism
The ability of a drug to decrease the effect of
the other one is called antagonism. It may be direct
or indirect also.
Conversely, drugs may have opposite actions, e.g. in asthmatic patients β‐blockers will oppose β‐agonists and may precipitate severe or even fatal asthma.
Most antagonists are drugs that bind to receptors but do not activate them. They may be competitive or irreversible. Other types of antagonist are less common.
Competitive antagonists bind reversibly with receptors, and the tissue response can be returned to normal by increasing the dose of agonist.
Слайд 56Irreversible antagonists have an effect that cannot be reversed by
increasing the concentration of agonist. The only important example is
phenoxybenzamine, which binds covalently with α‐adrenoceptors.
Non‐competitive antagonists do not bind to the receptor site but act downstream to prevent the response to an agonist, e.g. calcium‐channel blockers.
Chemical antagonists simply bind to the active drug and inactivate it; e.g. protamine abolishes the anticoagulant effect of heparin.
Physiological antagonists are two agents with opposite effects e.g. prostacyclin and thromboxane A2 on platelet aggregation.
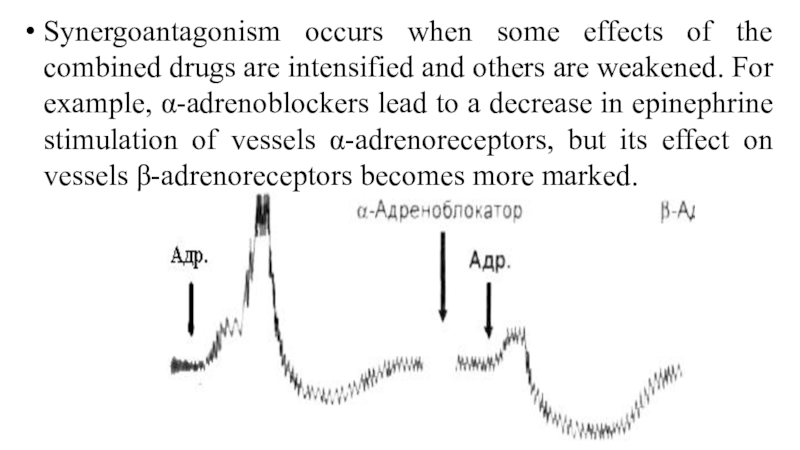
Слайд 57Synergoantagonism occurs when some effects of the combined drugs are
intensified and others are weakened. For example, α-adrenoblockers lead to
a decrease in epinephrine stimulation of vessels α-adrenoreceptors, but its effect on vessels β-adrenoreceptors becomes more marked.
Слайд 58Pharmacokinetic interactions
Absorption. Drugs that increase (e.g. metoclopramide) or decrease (e.g.
atropine) the rate of gastric emptying may affect absorption. Enterohepatic
recirculation of oral contraceptives (especially low‐dose oestrogen) may be decreased by antibiotics and lead to pregnancy (antibiotics kill the gut bacteria that normally release the steroid from the conjugated form excreted in bile).
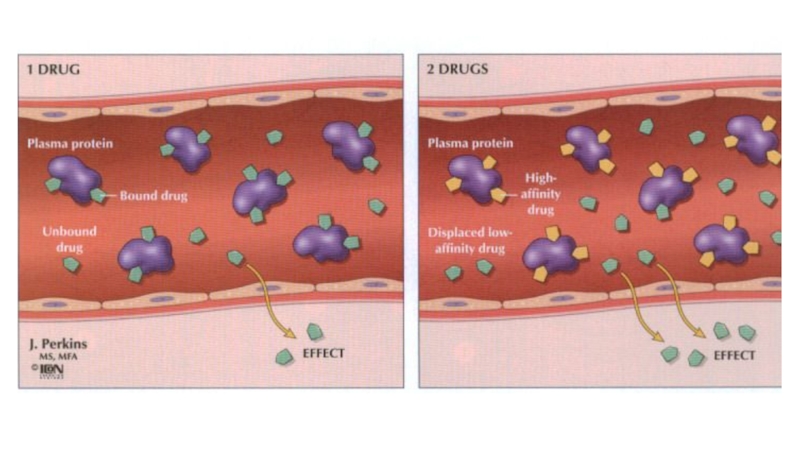
Distribution. Many drugs are bound to plasma albumin and may be displaced by a second drug.
Слайд 59Metabolism. Induction of hepatic enzymes by a second drug (e.g.phenobarbital,
rifampicin) can decrease the efficacy of drugs metabolized by the
same enzymes (e.g.warfarin). Enzyme inhibitors (e.g. cimetidine) potentiate the effects of warfarin and may cause phenytoin and theophylline toxicity.
Excretion. Drugs may share the same transport system in the proximal tubules. Thus, probenecid competitively reduces penicillin excretion. Potassium‐sparing diuretics combined with angiotensinconverting enzyme (ACE) inhibitors cause hyperkalaemia.
Слайд 60Adverse drug reactions
Adverse drug reactions can be divided into those
that are predictable and dose‐related (e.g. hypoglycaemia with insulin, bleeding
with warfarin) or sometimes a drug’s parallel unwanted action (e.g. respiratory depression with morphine).
and those that are unpredictable and not necessarily dose‐related (drug allergy or immunological reactions, idiosyncrasy).
Слайд 61Cumulation – storage of pharmacological substance in the body. It
is typical for slow-acting drugs, that are released slowly or
are steadily bound in the body. Cumulation after repeated administration may be the cause of toxic effects.
Renal disease can lead to accumulation and toxicity if a drug is excreted by glomerular filtration or tubular secretion (e.g. gentamicin and other aminoglycosides, digoxin, amphotericin, captopril). Liver disease can lead to accumulation of drugs metabolized in the liver.
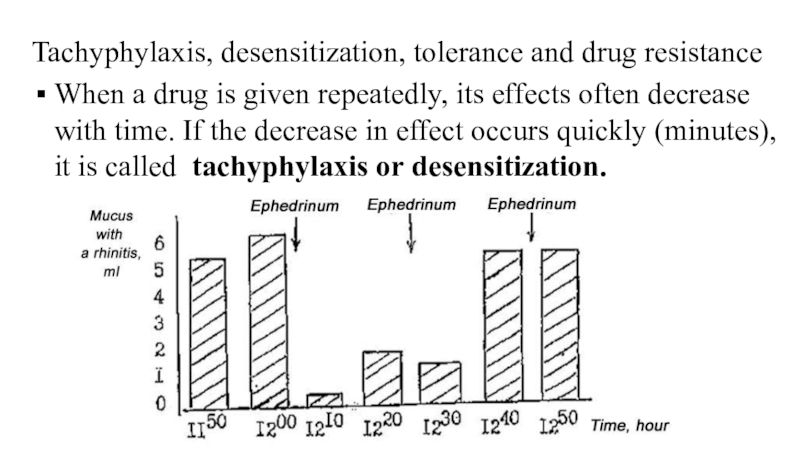
Слайд 62Tachyphylaxis, desensitization, tolerance and drug resistance
When a drug is given
repeatedly, its effects often decrease with time. If the decrease
in effect occurs quickly (minutes), it is called tachyphylaxis or desensitization.
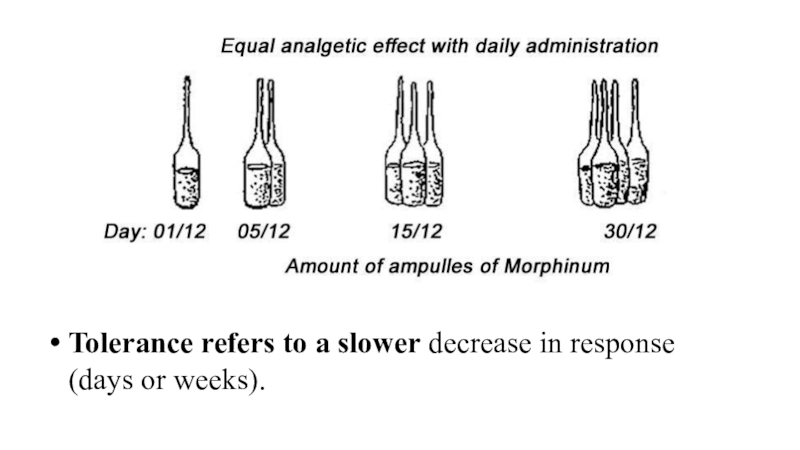
Слайд 63Tolerance refers to a slower decrease in response (days or
weeks).
Слайд 64Tolerance may involve increased metabolism of a drug, e.g. ethanol,
barbiturates, or homeostatic mechanisms (usually not understood) that gradually reduce
the effect of a drug, e.g. morphine. Changes in receptors may cause desensitization, e.g. suxamethonium. . A decrease in receptor number (downregulation) can lead to tolerance, e.g. insulin.
Drug resistance is a term reserved for the loss of effect of chemotherapeutic agents, e.g. antimalarials.
Слайд 65Drug dependence is a term used when a person has
a compulsion to take a drug in order to experience
its psychic effects, and sometimes to avoid the discomfort of withdrawal symptoms.
Psychological dependence: cocaine, LSD, psilocin. Cannabis has hallucinogenic action. It produces feelings of euphoria, relaxation and well‐being.
Physical dependence on opioid analgesics gradually develops, and sudden termination of drug administration precipitates a withdrawal syndrome (characterized by yawning, sweating, gooseflesh, tremor, irritability, anorexia, nausea and vomiting).
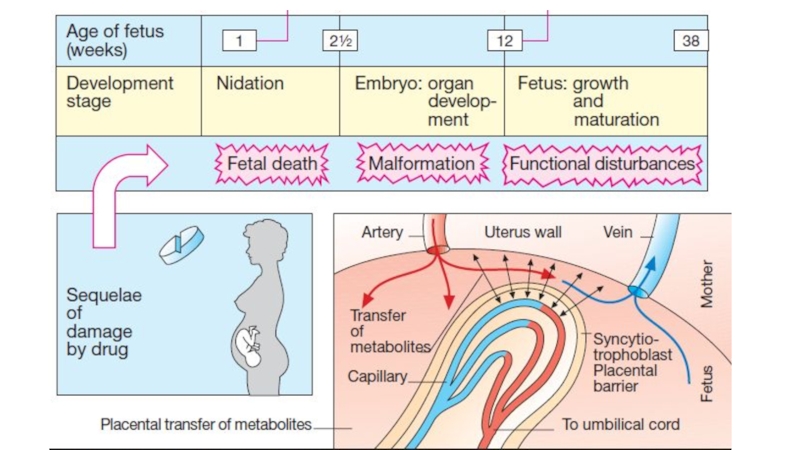
Слайд 66Teratogenesis is the occurrence of fetal developmental abnormalities caused by
drugs taken during the first trimester of pregnancy. Most drugs
cross the placental barrier to some extent and, if possible, drugs should be avoided during pregnancy.
Known teratogens include alcohol (fetal alcohol syndrome), anticancer drugs, warfarin (multiple congenital defects), valproate, carbamazepine (neural tube defects), and other anticonvulsants and tetracyclines (inhibition of bone growth).
Слайд 68Carcinogenesis. Drug‐induced tumours are probably very rare because the pharmaceutical
industry makes great efforts to avoid marketing carcinogenic agents. The
mechanisms involved in chemical carcinogenesis are usually unknown, but immunosuppression (e.g. azathioprine with prednisolone) is associated with a greatly increased risk of lymphomas.
Слайд 69Literature
1. Tripathi K.D. Essentials of Medical Pharmacology. Eighth Edition. -2019.-
Jaypee Brothers Medical Publishers. The Health Sciences Publisher. -New Delhi.
London. Panama
2. D.A.Kharkevich. Pharmacology. Textbook for medical students. Translation of 12th edition of Russion textbook “Pharmacology” (2017). – М., ГЭОТАР-Медиа, 2017.
3. Review of pharmacology. Gobind Rai Garg, Sparsh Gupta. 13th edition. - 2019.- Jaypee Brothers Medical Publishers. The Health Sciences Publisher. -New Delhi. London. Panama
4. Whalen Karen. Lippincott Illustrated Reviews: Pharmacology. Sixth Edition. - Wolters Kluwer. - 2015.-Philadelphia
5. Color Atlas of Pharmacology. 2nd edition, revised and expanded. Heinz Lüllmann.- 2000 Thieme
6. Pharmacology Examination & Board Review. Tenth Edition. Trevor Anthony J., Katzung Bertram G., Kruidering-Hall Marieke, Susan B. Masters. - a LANGE medical book. - 2013.-New York
7. Medical Pharmacology at a Glance. Eighth Edition. Neal Michael J. – 2016. John Wiley & Sons, Ltd.
8. USMLE Step 1. Lecture Notes. Pharmacology. Lionel P.Raymon and others.- Kaplan Medical.Inc. -2009