Разделы презентаций
- Разное
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Applications of nuclear physics
Содержание
- 1. Applications of nuclear physics
- 2. Interactions Involving NeutronsTo understand nuclear fission and
- 3. Interactions Involving NeutronsEventually, most neutrons bombarding a
- 4. Nuclear FissionA nuclear fission occurs when a
- 5. Nuclear FissionA nuclear fission event
- 6. Nuclear FissionDistribution of fission products versus mass
- 7. Nuclear ReactorsA nuclear chain reaction initiated by
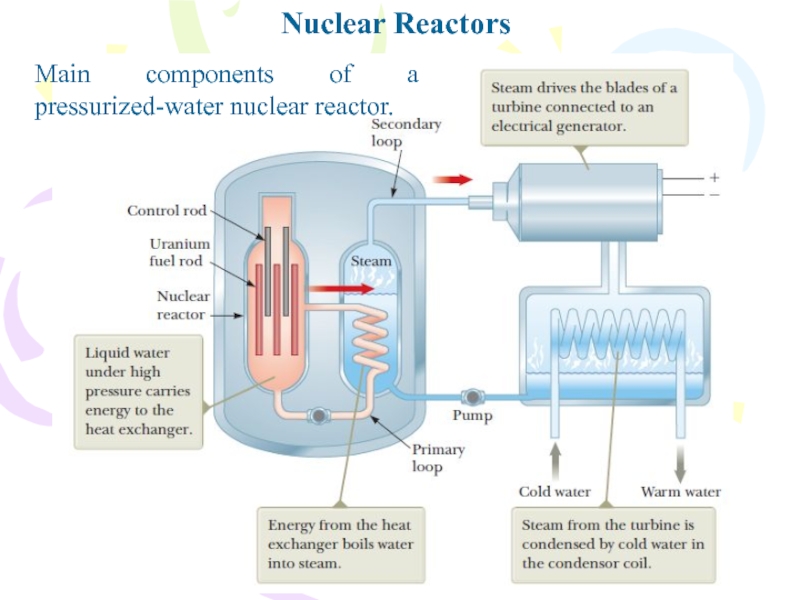
- 8. Nuclear ReactorsMain components of a pressurized-water nuclear reactor.
- 9. Nuclear ReactorsControl of Power LevelCross section of
- 10. Nuclear ReactorsSafety and Waste DisposalThese fusion reactions
- 11. Terrestrial Fusion ReactionsThe reactions that appear most
- 12. Nuclear FusionThe temperature at which the power
- 13. Nuclear FusionIn addition to the high-temperature requirements,
- 14. Nuclear FusionMagnetic ConfinementMany fusion-related plasma experiments use
- 15. Nuclear FusionMagnetic Confinement
- 16. Nuclear FusionInertial ConfinementThe second technique for confining
- 17. Nuclear FusionFusion Reactor DesignIn the D–T fusion
- 18. Nuclear FusionFusion Reactor DesignDiagram of a fusion reactor.
- 19. Advantages and Problems of FusionNuclear FusionIf fusion
- 20. Nuclear FusionAdvantages and Problems of FusionSeveral units
- 21. The RBE (relative biological effectiveness) factor for
- 22. Nuclear FusionAdvantages and Problems of FusionLow-level radiation
- 23. Radiation DetectorsParticles passing through matter interact with
- 24. Radiation DetectorsA photographic emulsion is the simplest example of a detector.
- 25. Radiation DetectorsIn an ion chamber, electron–ion pairs
- 26. Radiation DetectorsThe Geiger counter is perhaps the
- 27. Uses of RadiationTracingA tracer technique for determining the condition of the human circulatory system.
- 28. Uses of RadiationMaterials AnalysisFor centuries, a standard
- 29. Uses of RadiationRadiation TherapyRadiation causes much damage
- 30. Uses of RadiationFood PreservationRadiation is finding increasing
- 31. Скачать презентанцию
Слайды и текст этой презентации
Слайд 2Interactions Involving Neutrons
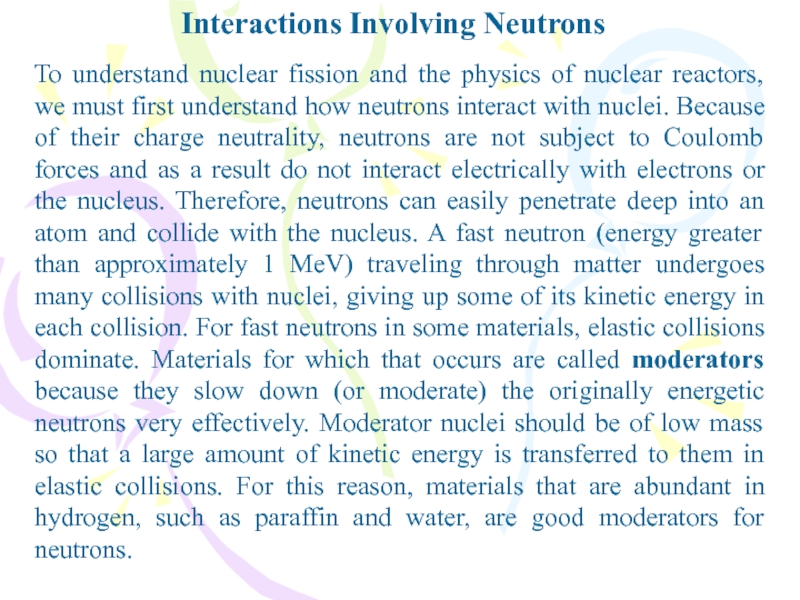
To understand nuclear fission and the physics of
nuclear reactors, we must first understand how neutrons interact with
nuclei. Because of their charge neutrality, neutrons are not subject to Coulomb forces and as a result do not interact electrically with electrons or the nucleus. Therefore, neutrons can easily penetrate deep into an atom and collide with the nucleus. A fast neutron (energy greater than approximately 1 MeV) traveling through matter undergoes many collisions with nuclei, giving up some of its kinetic energy in each collision. For fast neutrons in some materials, elastic collisions dominate. Materials for which that occurs are called moderators because they slow down (or moderate) the originally energetic neutrons very effectively. Moderator nuclei should be of low mass so that a large amount of kinetic energy is transferred to them in elastic collisions. For this reason, materials that are abundant in hydrogen, such as paraffin and water, are good moderators for neutrons.Слайд 3Interactions Involving Neutrons
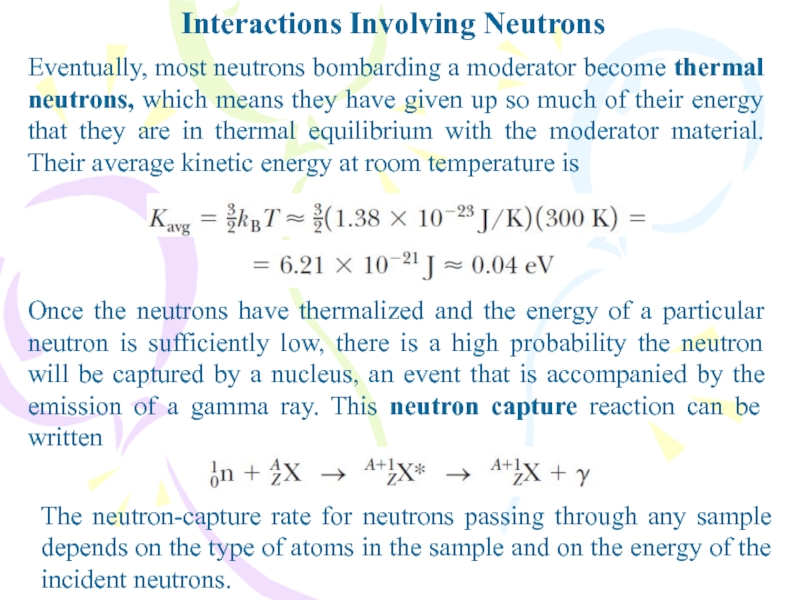
Eventually, most neutrons bombarding a moderator become thermal
neutrons, which means they have given up so much of
their energy that they are in thermal equilibrium with the moderator material. Their average kinetic energy at room temperature isOnce the neutrons have thermalized and the energy of a particular neutron is sufficiently low, there is a high probability the neutron will be captured by a nucleus, an event that is accompanied by the emission of a gamma ray. This neutron capture reaction can be written
The neutron-capture rate for neutrons passing through any sample depends on the type of atoms in the sample and on the energy of the incident neutrons.
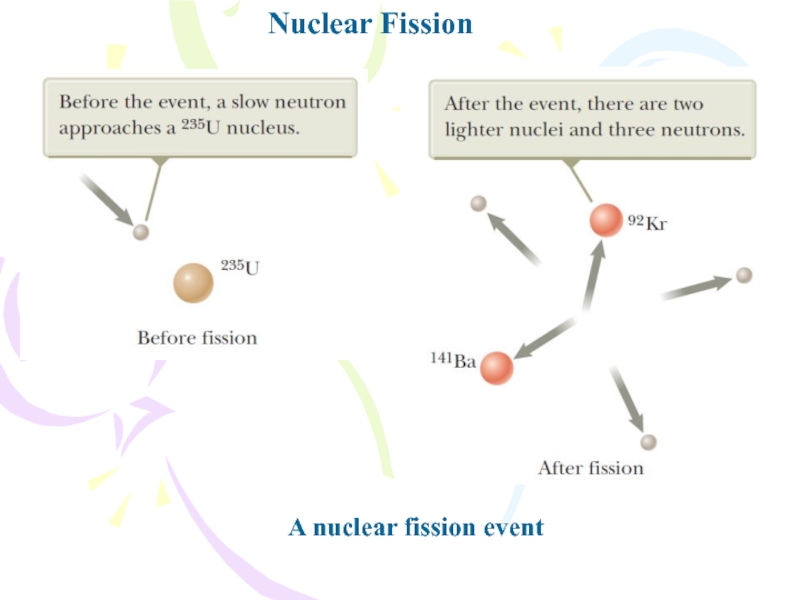
Слайд 4Nuclear Fission
A nuclear fission occurs when a heavy nucleus, such
as 235U, splits into two smaller nuclei.
The absorption of the
neutron creates a nucleus that is unstable and can change to a lower-energy configuration by splitting into two smaller nuclei. In such a reaction, the combined mass of the daughter nuclei is less than the mass of the parent nucleus, and the difference in mass is called the mass defect.where 236U* is an intermediate excited state that lasts for approximately 10-12 s before splitting into medium-mass nuclei X and Y, which are called fission fragments.
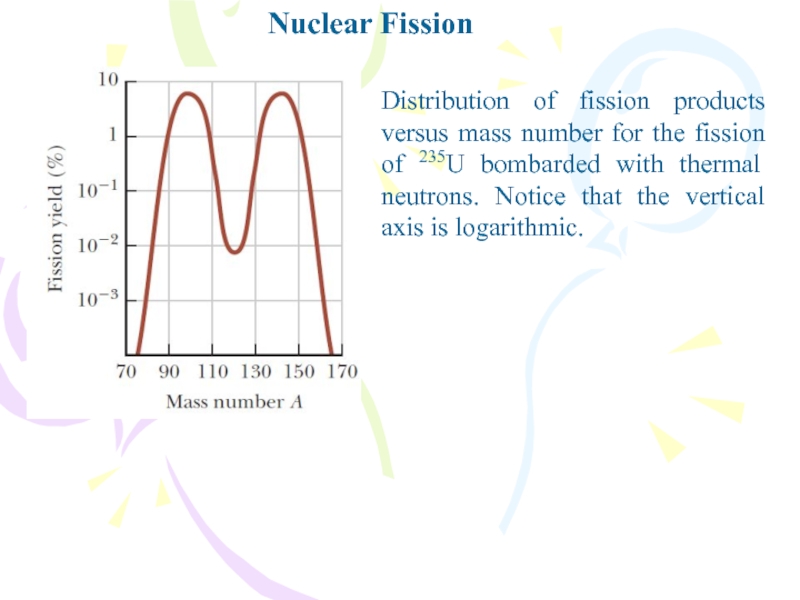
Слайд 6Nuclear Fission
Distribution of fission products versus mass number for the
fission of 235U bombarded with thermal neutrons. Notice that the
vertical axis is logarithmic.Слайд 7Nuclear Reactors
A nuclear chain reaction initiated by the capture of
a neutron. Uranium nuclei are shown in tan, neutrons in
gray, and daughter nuclei in orange.Слайд 9Nuclear Reactors
Control of Power Level
Cross section of a reactor core
showing the control rods, fuel elements containing enriched fuel, and
moderating material, all surrounded by a radiation shield.The basic design of a nuclear reactor core
Слайд 10Nuclear Reactors
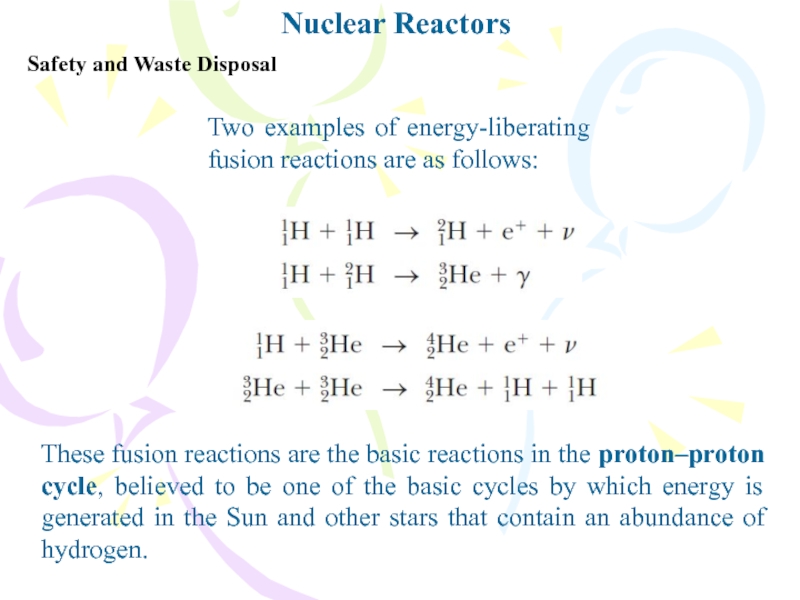
Safety and Waste Disposal
These fusion reactions are the basic
reactions in the proton–proton cycle, believed to be one of
the basic cycles by which energy is generated in the Sun and other stars that contain an abundance of hydrogen.Two examples of energy-liberating fusion reactions are as follows:
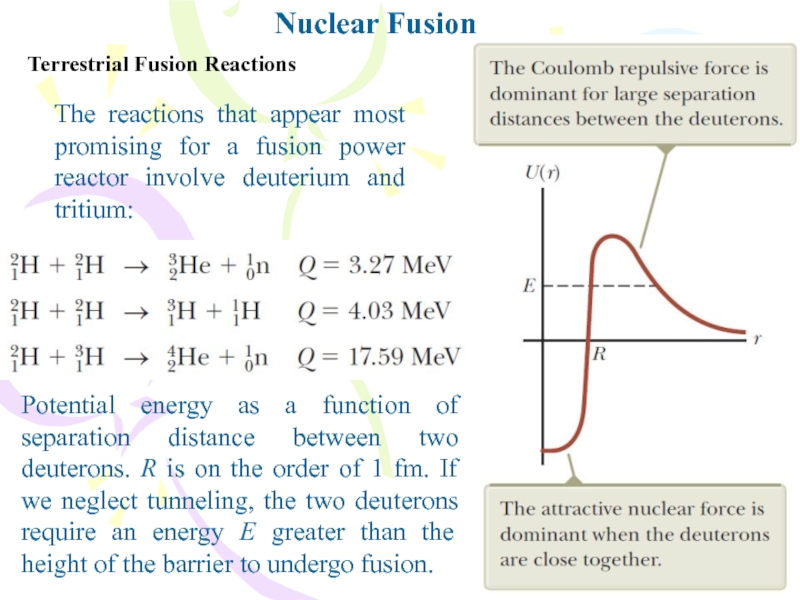
Слайд 11Terrestrial Fusion Reactions
The reactions that appear most promising for a
fusion power reactor involve deuterium and tritium:
Potential energy as a
function of separation distance between two deuterons. R is on the order of 1 fm. If we neglect tunneling, the two deuterons require an energy E greater than the height of the barrier to undergo fusion.Nuclear Fusion
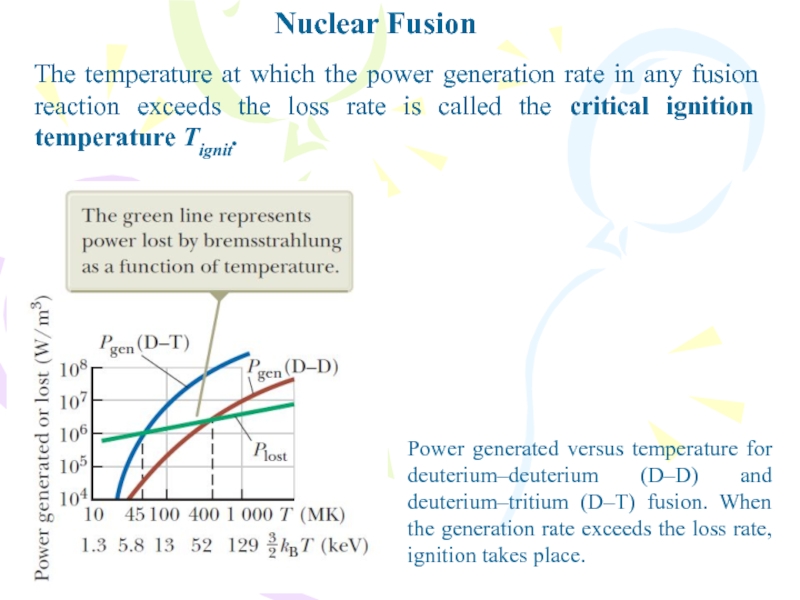
Слайд 12Nuclear Fusion
The temperature at which the power generation rate in
any fusion reaction exceeds the loss rate is called the
critical ignition temperature Tignit.Power generated versus temperature for deuterium–deuterium (D–D) and deuterium–tritium (D–T) fusion. When the generation rate exceeds the loss rate, ignition takes place.
Слайд 13Nuclear Fusion
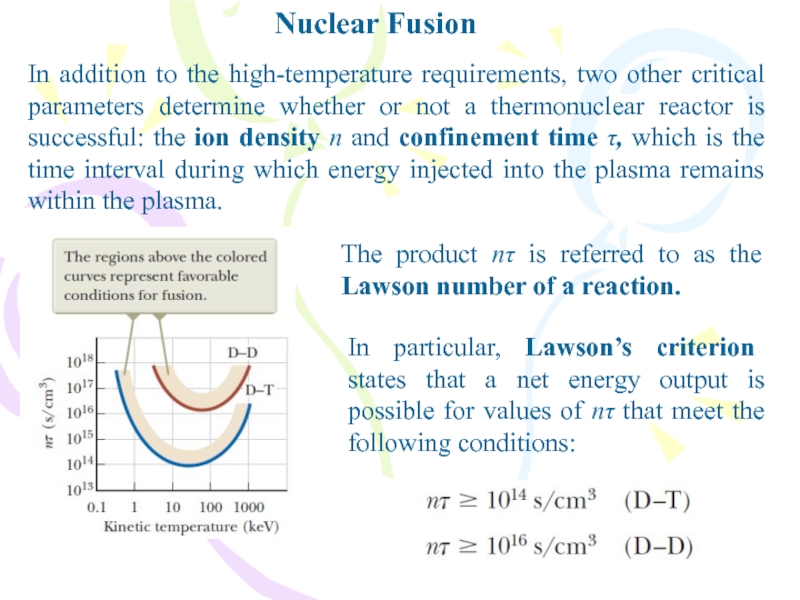
In addition to the high-temperature requirements, two other critical
parameters determine whether or not a thermonuclear reactor is successful:
the ion density n and confinement time τ, which is the time interval during which energy injected into the plasma remains within the plasma.The product nτ is referred to as the Lawson number of a reaction.
In particular, Lawson’s criterion states that a net energy output is possible for values of nτ that meet the following conditions:
Слайд 14Nuclear Fusion
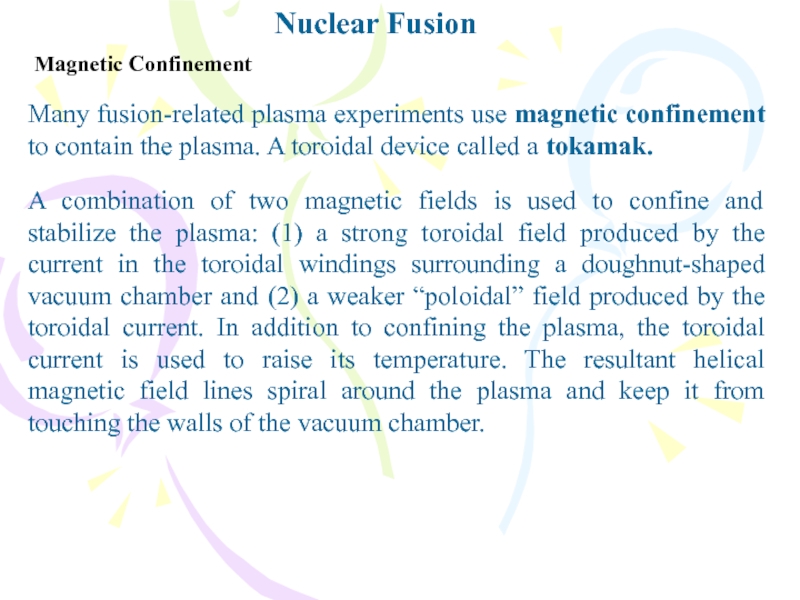
Magnetic Confinement
Many fusion-related plasma experiments use magnetic confinement to
contain the plasma. A toroidal device called a tokamak.
A combination
of two magnetic fields is used to confine and stabilize the plasma: (1) a strong toroidal field produced by the current in the toroidal windings surrounding a doughnut-shaped vacuum chamber and (2) a weaker “poloidal” field produced by the toroidal current. In addition to confining the plasma, the toroidal current is used to raise its temperature. The resultant helical magnetic field lines spiral around the plasma and keep it from touching the walls of the vacuum chamber.Слайд 16Nuclear Fusion
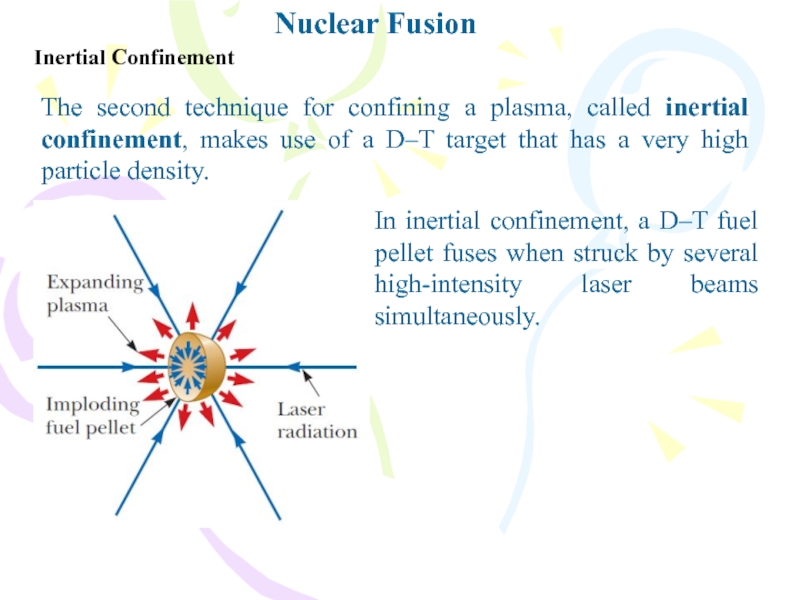
Inertial Confinement
The second technique for confining a plasma, called
inertial confinement, makes use of a D–T target that has
a very high particle density.In inertial confinement, a D–T fuel pellet fuses when struck by several high-intensity laser beams simultaneously.
Слайд 17Nuclear Fusion
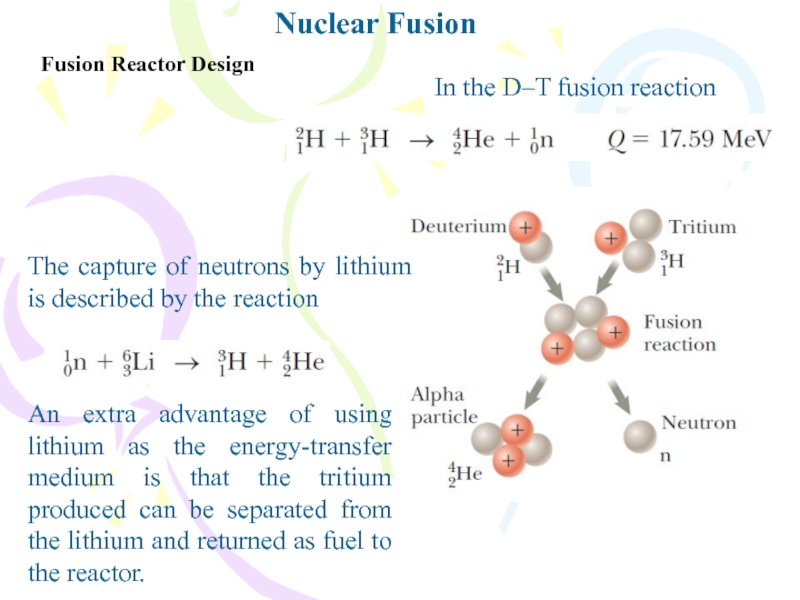
Fusion Reactor Design
In the D–T fusion reaction
The capture of
neutrons by lithium is described by the reaction
An extra advantage
of using lithium as the energy-transfer medium is that the tritium produced can be separated from the lithium and returned as fuel to the reactor.Слайд 19Advantages and Problems of Fusion
Nuclear Fusion
If fusion power can ever
be harnessed, it will offer several advantages over fissiongenerated power:
(1) low cost and abundance of fuel (deuterium), (2) impossibility of runaway accidents, and (3) decreased radiation hazard. Some of the anticipated problems and disadvantages include (1) scarcity of lithium, (2) limited supply of helium, which is needed for cooling the superconducting magnets used to produce strong confining fields, and (3) structural damage and induced radioactivity caused by neutron bombardment. If such problems and the engineering design factors can be resolved, nuclear fusion may become a feasible source of energy by the middle of the twenty-first century.Слайд 20Nuclear Fusion
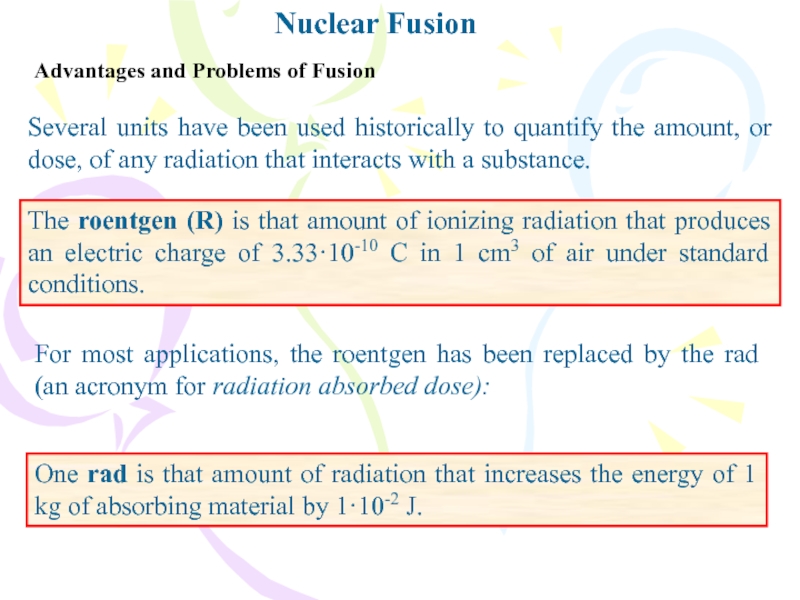
Advantages and Problems of Fusion
Several units have been used
historically to quantify the amount, or dose, of any radiation
that interacts with a substance.The roentgen (R) is that amount of ionizing radiation that produces an electric charge of 3.33·10-10 C in 1 cm3 of air under standard conditions.
For most applications, the roentgen has been replaced by the rad (an acronym for radiation absorbed dose):
One rad is that amount of radiation that increases the energy of 1 kg of absorbing material by 1·10-2 J.
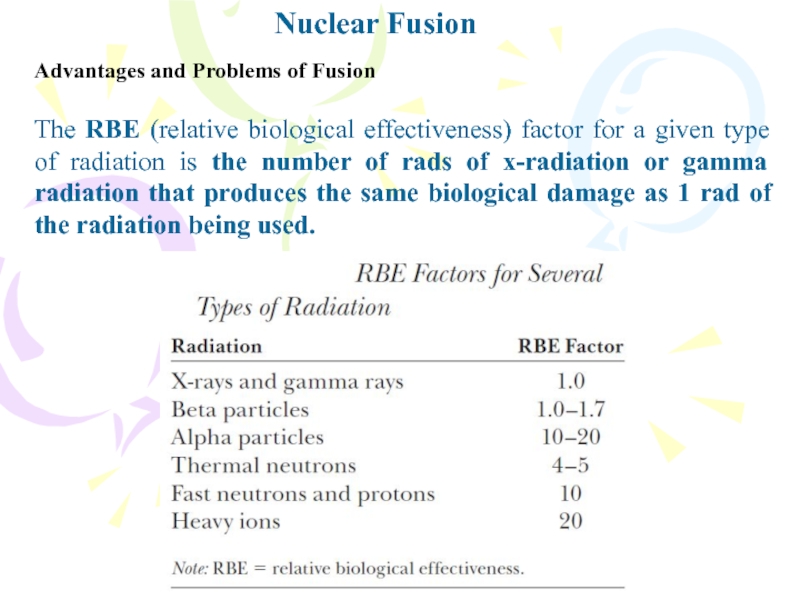
Слайд 21The RBE (relative biological effectiveness) factor for a given type
of radiation is the number of rads of x-radiation or
gamma radiation that produces the same biological damage as 1 rad of the radiation being used.Nuclear Fusion
Advantages and Problems of Fusion
Слайд 22Nuclear Fusion
Advantages and Problems of Fusion
Low-level radiation from natural sources
such as cosmic rays and radioactive rocks and soil delivers
to each of us a dose of approximately 0.13 rem/yr. This radiation, called background radiation, varies with geography, with the main factors being altitude (exposure to cosmic rays) and geology (radon gas released by some rock formations, deposits of naturally radioactive minerals).Слайд 23Radiation Detectors
Particles passing through matter interact with the matter in
several ways. The particle can, for example, ionize atoms, scatter
from atoms, or be absorbed by atoms. Radiation detectors exploit these interactions to allow a measurement of the particle’s energy, momentum, or charge and sometimes the very existence of the particle if it is otherwise difficult to detect. Various devices have been developed for detecting radiation. These devices are used for a variety of purposes, including medical diagnoses, radioactive dating measurements, measuring background radiation, and measuring the mass, energy, and momentum of particles created in high-energy nuclear reactions.Слайд 25Radiation Detectors
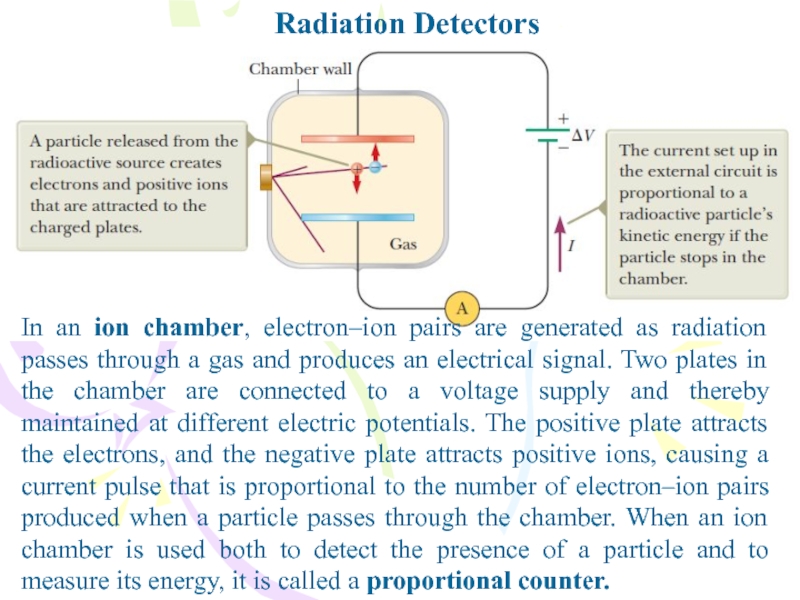
In an ion chamber, electron–ion pairs are generated as
radiation passes through a gas and produces an electrical signal.
Two plates in the chamber are connected to a voltage supply and thereby maintained at different electric potentials. The positive plate attracts the electrons, and the negative plate attracts positive ions, causing a current pulse that is proportional to the number of electron–ion pairs produced when a particle passes through the chamber. When an ion chamber is used both to detect the presence of a particle and to measure its energy, it is called a proportional counter.Слайд 26Radiation Detectors
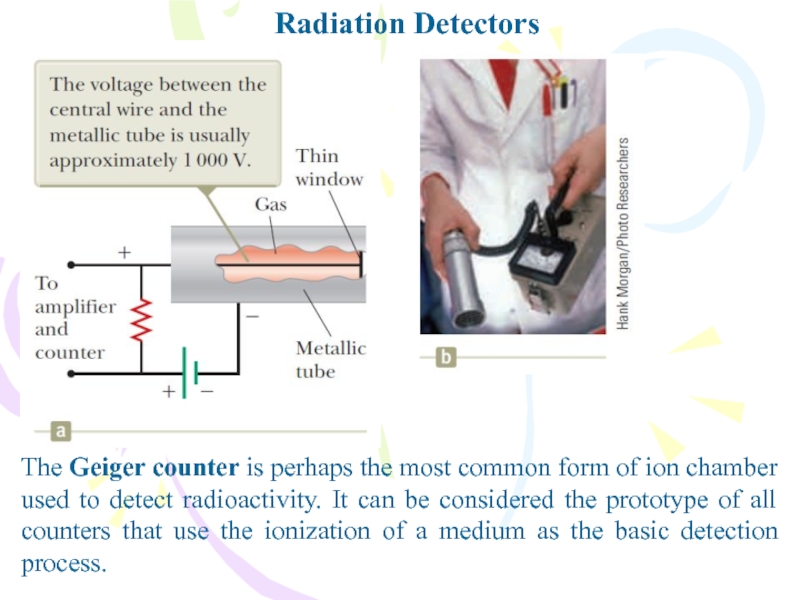
The Geiger counter is perhaps the most common form
of ion chamber used to detect radioactivity. It can be
considered the prototype of all counters that use the ionization of a medium as the basic detection process.Слайд 27Uses of Radiation
Tracing
A tracer technique for determining the condition of
the human circulatory system.
Слайд 28Uses of Radiation
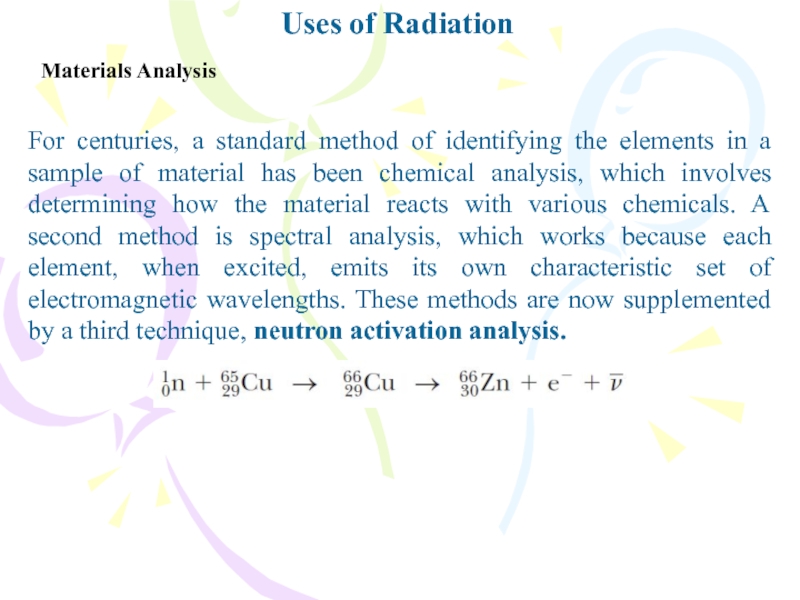
Materials Analysis
For centuries, a standard method of identifying
the elements in a sample of material has been chemical
analysis, which involves determining how the material reacts with various chemicals. A second method is spectral analysis, which works because each element, when excited, emits its own characteristic set of electromagnetic wavelengths. These methods are now supplemented by a third technique, neutron activation analysis.Слайд 29Uses of Radiation
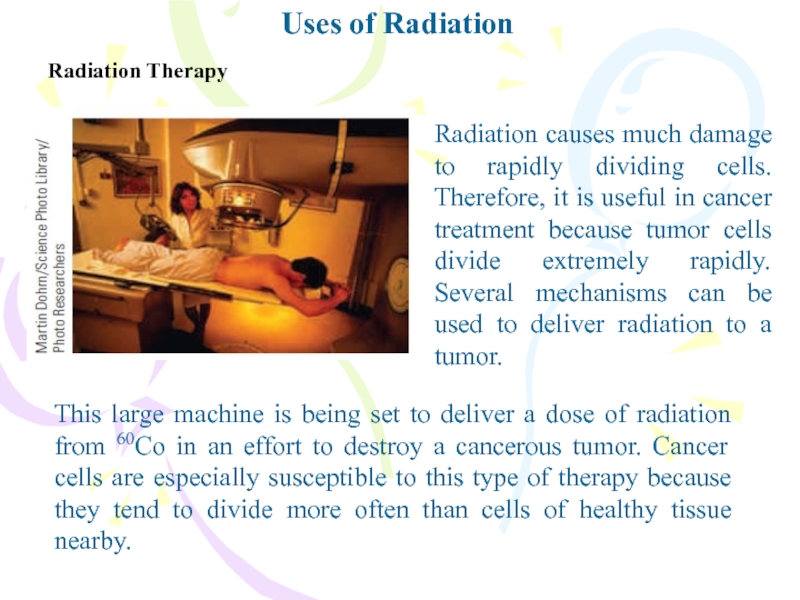
Radiation Therapy
Radiation causes much damage to rapidly dividing
cells. Therefore, it is useful in cancer treatment because tumor
cells divide extremely rapidly. Several mechanisms can be used to deliver radiation to a tumor.This large machine is being set to deliver a dose of radiation from 60Co in an effort to destroy a cancerous tumor. Cancer cells are especially susceptible to this type of therapy because they tend to divide more often than cells of healthy tissue nearby.
Слайд 30Uses of Radiation
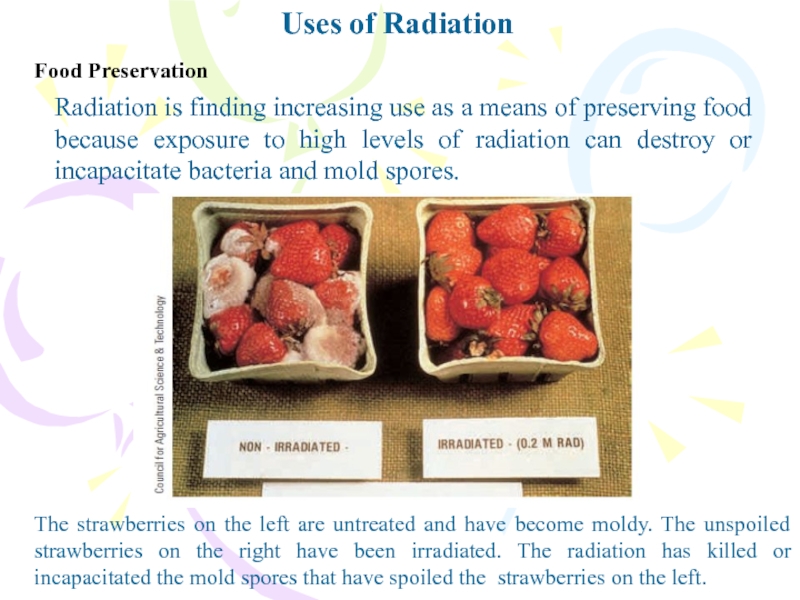
Food Preservation
Radiation is finding increasing use as a
means of preserving food because exposure to high levels of
radiation can destroy or incapacitate bacteria and mold spores.The strawberries on the left are untreated and have become moldy. The unspoiled strawberries on the right have been irradiated. The radiation has killed or incapacitated the mold spores that have spoiled the strawberries on the left.